Volatile Metabolic Markers for Monitoring Pectobacterium carotovorum subsp. carotovorum Using Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry
- PMID: 33203818
- PMCID: PMC9705696
- DOI: 10.4014/jmb.2009.09028
Volatile Metabolic Markers for Monitoring Pectobacterium carotovorum subsp. carotovorum Using Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry
Abstract
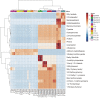
Identifying the extracellular metabolites of microorganisms in fresh vegetables is industrially useful for assessing the quality of processed foods. Pectobacterium carotovorum subsp. carotovorum (PCC) is a plant pathogenic bacterium that causes soft rot disease in cabbages. This microbial species in plant tissues can emit specific volatile molecules with odors that are characteristic of the host cell tissues and PCC species. In this study, we used headspace solid-phase microextraction followed by gas chromatography coupled with mass spectrometry (HS-SPME-GC-MS) to identify volatile compounds (VCs) in PCC-inoculated cabbage at different storage temperatures. HS-SPME-GC-MS allowed for recognition of extracellular metabolites in PCC-infected cabbages by identifying specific volatile metabolic markers. We identified 4-ethyl-5-methylthiazole and 3-butenyl isothiocyanate as markers of fresh cabbages, whereas 2,3-butanediol and ethyl acetate were identified as markers of soft rot in PCC-infected cabbages. These analytical results demonstrate a suitable approach for establishing non-destructive plant pathogen-diagnosis techniques as alternatives to standard methods, within the framework of developing rapid and efficient analytical techniques for monitoring plant-borne bacterial pathogens. Moreover, our techniques could have promising applications in managing the freshness and quality control of cabbages.
Keywords: Cabbage; Pectobacterium; soft rot; solid-phase microextraction; volatile metabolic marker.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures





References
-
- Morath SU, Hung R, Bennett JW. Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012;26:73–83. doi: 10.1016/j.fbr.2012.07.001. - DOI
-
- Strobel G. Muscodor species-endophytes with biological promise. Phytochem. Rev. 2011;10:165–172. doi: 10.1007/s11101-010-9163-3. - DOI
-
- Li Q, Ning P, Zheng L, Huang J, Li G, Hsiang T. Effects of volatile substances of Streptomyces globisporus JK-1 on control of Botrytis cinerea on tomato fruit. Biol. Control. 2012;61:113–120. doi: 10.1016/j.biocontrol.2011.10.014. - DOI
-
- Zheng M, Shi J, Shi J, Wang Q, Li Y. Antimicrobial effects of volatiles produced by two antagonistic Bacillus strains on the anthracnose pathogen in postharvest mangos. Biol. Control. 2013;65:200–206. doi: 10.1016/j.biocontrol.2013.02.004. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

