Engineered B cells expressing an anti-HIV antibody enable memory retention, isotype switching and clonal expansion
- PMID: 33203857
- PMCID: PMC7673991
- DOI: 10.1038/s41467-020-19649-1
Engineered B cells expressing an anti-HIV antibody enable memory retention, isotype switching and clonal expansion
Abstract
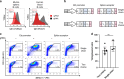
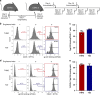
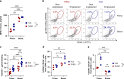
HIV viremia can be controlled by chronic antiretroviral therapy. As a potentially single-shot alternative, B cells engineered by CRISPR/Cas9 to express anti-HIV broadly neutralizing antibodies (bNAbs) are capable of secreting high antibody titers. Here, we show that, upon immunization of mice, adoptively transferred engineered B cells home to germinal centers (GC) where they predominate over the endogenous response and differentiate into memory and plasma cells while undergoing class switch recombination (CSR). Immunization with a high affinity antigen increases accumulation in GCs and CSR rates. Boost immunization increases the rate of engineered B cells in GCs and antibody secretion, indicating memory retention. Finally, antibody sequences of engineered B cells in the spleen show patterns of clonal selection. Therefore, B cells can be engineered into what could be a living and evolving drug.
Conflict of interest statement
A.D.N., M.H.-F., T.A., D.N., I.D. and A.B. declare that they are listed as inventors on relevant patent applications. The other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

