Hollow silica reinforced magnesium nanocomposites with enhanced mechanical and biological properties with computational modeling analysis for mandibular reconstruction
- PMID: 33203862
- PMCID: PMC7673133
- DOI: 10.1038/s41368-020-00098-x
Hollow silica reinforced magnesium nanocomposites with enhanced mechanical and biological properties with computational modeling analysis for mandibular reconstruction
Abstract
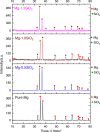
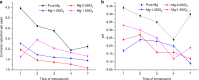
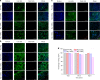
The present study investigates Mg-SiO2 nanocomposites as biodegradable implants for orthopedic and maxillofacial applications. The effect of presence and progressive addition of hollow silica nanoparticles (0.5, 1, and 1.5) vol.% on the microstructural, mechanical, degradation, and biocompatibility response of pure Mg were investigated. Results suggest that the increased addition of hollow silica nanoparticles resulted in a progressive increase in yield strength and ultimate compressive strength with Mg-1.5 vol.% SiO2 exhibiting superior enhancement. The response of Mg-SiO2 nanocomposites under the influence of Hanks' balanced salt solution revealed that the synthesized composites revealed lower corrosion rates, indicating rapid dynamic passivation when compared with pure Mg. Furthermore, cell adhesion and proliferation of osteoblast cells were noticeably higher than pure Mg with the addition of 1 vol.% SiO2 nanoparticle. The biocompatibility and the in vitro biodegradation of the Mg-SiO2 nanocomposites were influenced by the SiO2 content in pure Mg with Mg-0.5 vol.% SiO2 nanocomposite exhibiting the best corrosion resistance and biocompatibility when compared with other nanocomposites. Enhancement in mechanical, corrosion, and biocompatibility characteristics of Mg-SiO2 nanocomposites developed in this study are also compared with properties of other metallic biomaterials used in alloplastic mandibular reconstruction in a computational model.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Prasadh S, et al. The potential of magnesium based materials in mandibular reconstruction. Metals. 2019;9:302. doi: 10.3390/met9030302. - DOI
-
- Ali M, Hussein MA, Al-Aqeeli N. Magnesium-based composites and alloys for medical applications: a review of mechanical and corrosion properties. J. Alloy. Compd. 2019;792:1162–1190. doi: 10.1016/j.jallcom.2019.04.080. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

