Understanding complex dynamics of behavioral, neurochemical and transcriptomic changes induced by prolonged chronic unpredictable stress in zebrafish
- PMID: 33203921
- PMCID: PMC7673038
- DOI: 10.1038/s41598-020-75855-3
Understanding complex dynamics of behavioral, neurochemical and transcriptomic changes induced by prolonged chronic unpredictable stress in zebrafish
Abstract
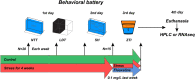
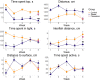
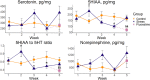
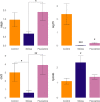
Stress-related neuropsychiatric disorders are widespread, debilitating and often treatment-resistant illnesses that represent an urgent unmet biomedical problem. Animal models of these disorders are widely used to study stress pathogenesis. A more recent and historically less utilized model organism, the zebrafish (Danio rerio), is a valuable tool in stress neuroscience research. Utilizing the 5-week chronic unpredictable stress (CUS) model, here we examined brain transcriptomic profiles and complex dynamic behavioral stress responses, as well as neurochemical alterations in adult zebrafish and their correction by chronic antidepressant, fluoxetine, treatment. Overall, CUS induced complex neurochemical and behavioral alterations in zebrafish, including stable anxiety-like behaviors and serotonin metabolism deficits. Chronic fluoxetine (0.1 mg/L for 11 days) rescued most of the observed behavioral and neurochemical responses. Finally, whole-genome brain transcriptomic analyses revealed altered expression of various CNS genes (partially rescued by chronic fluoxetine), including inflammation-, ubiquitin- and arrestin-related genes. Collectively, this supports zebrafish as a valuable translational tool to study stress-related pathogenesis, whose anxiety and serotonergic deficits parallel rodent and clinical studies, and genomic analyses implicate neuroinflammation, structural neuronal remodeling and arrestin/ubiquitin pathways in both stress pathogenesis and its potential therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Modeling consequences of prolonged strong unpredictable stress in zebrafish: Complex effects on behavior and physiology.Prog Neuropsychopharmacol Biol Psychiatry. 2018 Feb 2;81:384-394. doi: 10.1016/j.pnpbp.2017.08.021. Epub 2017 Aug 26. Prog Neuropsychopharmacol Biol Psychiatry. 2018. PMID: 28847526
-
Modulation of behavioral and neurochemical responses of adult zebrafish by fluoxetine, eicosapentaenoic acid and lipopolysaccharide in the prolonged chronic unpredictable stress model.Sci Rep. 2021 Jul 12;11(1):14289. doi: 10.1038/s41598-021-92422-6. Sci Rep. 2021. PMID: 34253753 Free PMC article.
-
Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish.Behav Brain Res. 2009 Dec 14;205(1):38-44. doi: 10.1016/j.bbr.2009.06.022. Epub 2009 Jun 18. Behav Brain Res. 2009. PMID: 19540270 Free PMC article.
-
Towards translational modeling of behavioral despair and its treatment in zebrafish.Behav Brain Res. 2022 Jul 26;430:113906. doi: 10.1016/j.bbr.2022.113906. Epub 2022 Apr 27. Behav Brain Res. 2022. PMID: 35489477 Review.
-
The evolutionarily conserved role of melatonin in CNS disorders and behavioral regulation: Translational lessons from zebrafish.Neurosci Biobehav Rev. 2019 Apr;99:117-127. doi: 10.1016/j.neubiorev.2018.12.025. Epub 2019 Jan 3. Neurosci Biobehav Rev. 2019. PMID: 30611799 Review.
Cited by
-
Teleosts as behaviour test models for social stress.Front Behav Neurosci. 2023 Sep 7;17:1205175. doi: 10.3389/fnbeh.2023.1205175. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37744951 Free PMC article. Review.
-
Investigating in vivo Mycobacterium avium subsp. paratuberculosis microevolution and mixed strain infections.Microbiol Spectr. 2023 Aug 16;11(5):e0171623. doi: 10.1128/spectrum.01716-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37584606 Free PMC article.
-
Experimental PTSD Models in Zebrafish: A Systematic Review of Behavioral, Neurochemical, and Molecular Outcomes.Biology (Basel). 2025 Apr 23;14(5):456. doi: 10.3390/biology14050456. Biology (Basel). 2025. PMID: 40427646 Free PMC article. Review.
-
Prolonged 5-week and 12-week chronic stress differentially modulates CNS expression of pro- and anti-neuroinflammatory biomarkers, brain monoamines and affective behavior in adult zebrafish.J Comp Physiol B. 2025 Jun;195(3):305-321. doi: 10.1007/s00360-025-01613-4. Epub 2025 Apr 12. J Comp Physiol B. 2025. PMID: 40220038
-
Evolutionarily conserved gene expression patterns for affective disorders revealed using cross-species brain transcriptomic analyses in humans, rats and zebrafish.Sci Rep. 2022 Dec 2;12(1):20836. doi: 10.1038/s41598-022-22688-x. Sci Rep. 2022. PMID: 36460699 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

