Corticotropin-releasing factor infusion in the bed nucleus of the stria terminalis of lactating mice alters maternal care and induces behavioural phenotypes in offspring
- PMID: 33204022
- PMCID: PMC7672063
- DOI: 10.1038/s41598-020-77118-7
Corticotropin-releasing factor infusion in the bed nucleus of the stria terminalis of lactating mice alters maternal care and induces behavioural phenotypes in offspring
Abstract
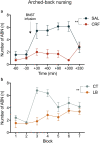
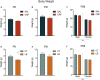
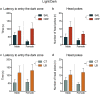
The peripartum period is accompanied by numerous physiological and behavioural adaptations organised by the maternal brain. These changes are essential for adequate expression of maternal behaviour, thereby ensuring proper development of the offspring. The corticotropin-releasing factor (CRF) plays a key role in a variety of behaviours accompanying stress, anxiety, and depression. There is also evidence that CRF contributes to maladaptations during the peripartum period. We investigated the effects of CRF in the bed nucleus of the stria terminalis (BNST) of lactating mice during maternal care and analysed locomotor activity and anxiety-like behaviour in the offspring. The BNST has been implicated in anxiety behaviour and regulation of the stress response. The effects of intra-BNST CRF administration were compared with those induced by the limited bedding (LB) procedure, a model that produces altered maternal behaviour. BALB/cJ dams were exposed to five infusions of CRF or saline into the BNST in the first weeks after birth while the LB dams were exposed to limited nesting material from postnatal days (P) 2-9. Maternal behaviour was recorded in intercalated days, from P1-9. Offspring anxiety-like behaviour was assessed during adulthood using the open-field, elevated plus-maze, and light/dark tests. Both intra-BNST CRF and LB exposure produced altered maternal care, represented by decreased arched-back nursing and increased frequency of exits from the nest. These changes in maternal care resulted in robust sex-based differences in the offspring's behavioural responses during adulthood. Females raised by CRF-infused dams exhibited increased anxiety-like behaviour, whereas males presented a significant decrease in anxiety. On the other hand, both males and females raised by dams exposed to LB showed higher locomotor activity. Our study demonstrates that maternal care is impaired by intra-BNST CRF administrations, and these maladaptations are similar to exposure to adverse early environments. These procedures, however, produce distinct phenotypes in mice during young adulthood and suggest sex-based differences in the susceptibility to poor maternal care.
Conflict of interest statement
The authors declare no competing interests.
Figures