Deep learning in cancer pathology: a new generation of clinical biomarkers
- PMID: 33204028
- PMCID: PMC7884739
- DOI: 10.1038/s41416-020-01122-x
Deep learning in cancer pathology: a new generation of clinical biomarkers
Abstract
Clinical workflows in oncology rely on predictive and prognostic molecular biomarkers. However, the growing number of these complex biomarkers tends to increase the cost and time for decision-making in routine daily oncology practice; furthermore, biomarkers often require tumour tissue on top of routine diagnostic material. Nevertheless, routinely available tumour tissue contains an abundance of clinically relevant information that is currently not fully exploited. Advances in deep learning (DL), an artificial intelligence (AI) technology, have enabled the extraction of previously hidden information directly from routine histology images of cancer, providing potentially clinically useful information. Here, we outline emerging concepts of how DL can extract biomarkers directly from histology images and summarise studies of basic and advanced image analysis for cancer histology. Basic image analysis tasks include detection, grading and subtyping of tumour tissue in histology images; they are aimed at automating pathology workflows and consequently do not immediately translate into clinical decisions. Exceeding such basic approaches, DL has also been used for advanced image analysis tasks, which have the potential of directly affecting clinical decision-making processes. These advanced approaches include inference of molecular features, prediction of survival and end-to-end prediction of therapy response. Predictions made by such DL systems could simplify and enrich clinical decision-making, but require rigorous external validation in clinical settings.
Conflict of interest statement
J.N.K. and N.T.R. have an unpaid advisory role at Pathomix (Heidelberg, Germany). The remaining authors declare no competing interests.
Figures

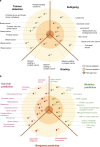
References
-
- Petrelli F, Ghidini M, Cabiddu M, Pezzica E, Corti D, Turati L, et al. Microsatellite instability and survival in stage II colorectal cancer: a systematic review and meta-analysis. Anticancer Res. 2019;39:6431–6441. - PubMed
-
- Naito Y, Urasaki T. Precision medicine in breast cancer. Chin. Clin. Oncol. 2018;7:29. - PubMed
-
- Mayekar MK, Bivona TG. Current landscape of targeted therapy in lung cancer. Clin. Pharmacol. Ther. 2017;102:757–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 771083/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- SFB CRC1382/P01, SFB-TRR57/P06, LU 1360/3-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 70113864/Deutsche Krebshilfe (German Cancer Aid)
- 691906/RWTH Aachen University (Rheinisch-Westfälische Technische Hochschule Aachen)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

