In Vitro and In Vivo Rat Model Assessments of the Effects of Vonoprazan on the Pharmacokinetics of Venlafaxine
- PMID: 33204067
- PMCID: PMC7667002
- DOI: 10.2147/DDDT.S276704
In Vitro and In Vivo Rat Model Assessments of the Effects of Vonoprazan on the Pharmacokinetics of Venlafaxine
Abstract
Purpose: The purpose of the present study was to investigate the effects of vonoprazan on the pharmacokinetics of venlafaxine in vitro and in vivo.
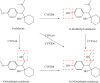
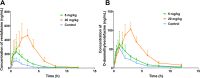
Methods: The mechanism underlying the inhibitory effect of vonoprazan on venlafaxine was investigated using rat liver microsomes. In vitro, the inhibition was evaluated by determining the production of O-desmethylvenlafaxine. Eighteen male Sprague-Dawley rats were randomly divided into three groups: control group, vonoprazan (5 mg/kg) group, and vonoprazan (20 mg/kg) group. A single dose of 20 mg/kg venlafaxine was administrated to rats orally without or with vonoprazan. Plasma was prepared from blood samples collected via the tail vein at different time points and concentrations of venlafaxine and its metabolite, O-desmethylvenlafaxine, were determined by ultra-performance liquid chromatography-tandem mass spectrometry.
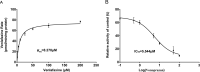
Results: We observed that vonoprazan could significantly decrease the amount of O-desmethylvenlafaxine (IC50 = 5.544 μM). Vonoprazan inhibited the metabolism of venlafaxine by a mixed inhibition, combining competitive and non-competitive inhibitory mechanisms. Compared with that in the control group (without vonoprazan), the pharmacokinetic parameters of venlafaxine and its metabolite, O-desmethylvenlafaxine, were significantly increased in both 5 and 20 mg/kg vonoprazan groups, with an increase in MRO-desmethylvenlafaxine.
Conclusion: Vonoprazan significantly alters the pharmacokinetics of venlafaxine in vitro and in vivo. Further investigations should be conducted to check these effects in humans. Therapeutic drug monitoring of venlafaxine in individuals undergoing venlafaxine maintenance therapy is recommended when vonoprazan is used concomitantly.
Keywords: gastroduodenal ulcer; gastroesophageal reflux disease; proton pump inhibitors; vonoprazan fumarate.
© 2020 Chen et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a Phase III, randomised, double-blind study. Gut. 2016;65(9):1439–1446. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

