Indole: The After Next Scaffold of Antiplasmodial Agents?
- PMID: 33204071
- PMCID: PMC7666986
- DOI: 10.2147/DDDT.S278588
Indole: The After Next Scaffold of Antiplasmodial Agents?
Abstract
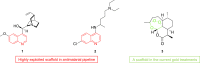
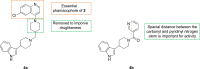
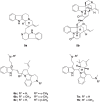
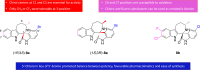
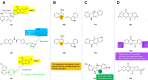
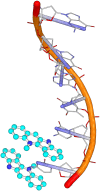
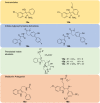
Malaria remains a global public health problem due to the uphill fight against the causative Plasmodium parasites that are relentless in developing resistance. Indole-based antiplasmodial compounds are endowed with multiple modes of action, of which inhibition of hemozoin formation is the major mechanism of action reported for compounds such as cryptolepine, flinderoles, and isosungucine. Indole-based compounds exert their potent activity against chloroquine-resistant Plasmodium strains by inhibiting hemozoin formation in a mode of action different from that of chloroquine or through a novel mechanism of action. For example, dysregulating the sodium and osmotic homeostasis of Plasmodium through inhibition of PfATP4 is the novel mechanism of cipargamin. The potential of developing multi-targeted compounds through molecular hybridization ensures the existence of indole-based compounds in the antimalarial pipeline.
Keywords: PfATP4; antimalarial agents; hemozoin inhibition; indole; multi-target approach.
© 2020 Surur et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World malaria report 2019. 2019. Available from: https://www.who.int/news-room/feature-stories/detail/world-malaria-repor.... Accessed October13, 2020.
-
- World malaria report 2018. 2019. Available from: https://www.mmv.org/newsroom/publications/world-malaria-report-2018. Accessed October13, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

