Intraclass Difference in Pneumonia Risk with Fluticasone and Budesonide in COPD: A Systematic Review of Evidence from Direct-Comparison Studies
- PMID: 33204085
- PMCID: PMC7667513
- DOI: 10.2147/COPD.S269637
Intraclass Difference in Pneumonia Risk with Fluticasone and Budesonide in COPD: A Systematic Review of Evidence from Direct-Comparison Studies
Abstract
Background: Inhaled corticosteroids (ICS) are widely used and recommended to treat chronic obstructive pulmonary disease (COPD). While generally considered safe, several studies demonstrated an increased risk of pneumonia with the use of ICS in COPD patients. Although all ICS indicated for COPD carry the class labeling warning of increased pneumonia risk, evidence suggests an intraclass difference in the risk of pneumonia between inhaled budesonide and fluticasone. To date, systematic reviews of direct-comparison studies have not been performed to assess if an intraclass difference exists.
Research question: This review investigated whether there is an intraclass difference in risk of pneumonia between inhaled fluticasone and budesonide, the 2 most commonly used ICS in COPD.
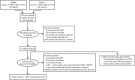
Study design and methods: A search of the medical literature was conducted in PubMed and Embase for the time period of 01/01/69-05/31/19. The search strategy combined terms that defined the patient/disease type, exposures, outcome, and the study/publication type. Descriptive and comparative statistics reported for fluticasone- and budesonide-containing products in each study, including data for pneumonia event subgroups, were extracted and reported by dose, seriousness, or practice setting. Controlled clinical trials and observational studies meeting the inclusion criteria were assessed for methodologic quality by using the appropriate tool from the list of study quality assessment tools developed by the National Institutes of Health.
Results: The summary relative risk (RR) ratio across 5 included studies (57,199 patients) was 1.13 (95% CI: 1.09-1.19), representing a 13.5% increased risk of pneumonia among fluticasone users compared to budesonide users. Similarly, summary RR ratio for serious pneumonia implied a 14.4% increased risk of serious pneumonia among fluticasone users compared to budesonide users (pooled RR: 1.14; 95% CI: 1.09-1.20).
Interpretation: There is likely a clinically important intraclass difference in the risk of pneumonia between fluticasone- and budesonide-containing inhaled medications in COPD.
Keywords: COPD; inhaled corticosteroids; pneumonia.
© 2020 Lodise et al.
Conflict of interest statement
Jingyi Li and Hitesh Gandhi are employees of AstraZeneca. Gerald O’Brien was an employee of AstraZeneca at the time of this study; he is now affiliated with Liquidia Technologies, Morrisville, NC. Thomas P Lodise is a consultant for AstraZeneca. Dr. Sethi has received research funding (to Jacobs School of Medicine, University at Buffalo, State University of New York) from Cipla, Sanofi, and GlaxoSmithKline; has been a consultant and/or participated in advisory boards for AstraZeneca, Boehringer Ingelheim, Circassia, Gilead, GlaxoSmithKline, Merck, Nabriva, Novavax, Paratek, Sunovion, and Theravance; has been a consultant for Aradigm and Pulmonx; and has been a speaker for AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. He has received royalties from UpToDate and Taylor and Francis. The authors report no other conflicts of interest in this work.
Figures
Comment in
-
Response to the Letter to the Editor Regarding "Intraclass Difference in Pneumonia Risk with Fluticasone and Budesonide in COPD: A Systematic Review of Evidence from Direct-Comparison Studies" [Response to Letter].Int J Chron Obstruct Pulmon Dis. 2021 May 10;16:1227-1229. doi: 10.2147/COPD.S315195. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34007165 Free PMC article. No abstract available.
-
Reply to: "Intraclass Difference in Pneumonia Risk with Fluticasone and Budesonide in COPD: A Systematic Review of Evidence from Direct-Comparison Studies" [Letter].Int J Chron Obstruct Pulmon Dis. 2021 May 10;16:1299-1301. doi: 10.2147/COPD.S304368. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34007169 Free PMC article. No abstract available.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report). 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical