AhR activation attenuates calcium oxalate nephrocalcinosis by diminishing M1 macrophage polarization and promoting M2 macrophage polarization
- PMID: 33204326
- PMCID: PMC7667681
- DOI: 10.7150/thno.51144
AhR activation attenuates calcium oxalate nephrocalcinosis by diminishing M1 macrophage polarization and promoting M2 macrophage polarization
Abstract
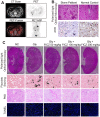
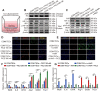
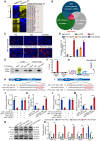
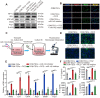
Calcium oxalate (CaOx) crystal can trigger kidney injury, which contributes to the pathogenesis of nephrocalcinosis. The phenotypes of infiltrating macrophage may impact CaOx-mediated kidney inflammatory injury as well as crystal deposition. How aryl hydrocarbon receptor (AhR) regulates inflammation and macrophage polarization is well understood; however, how it modulates CaOx nephrocalcinosis remains unclear. Methods: Mice were intraperitoneally injected with glyoxylate to establish CaOx nephrocalcinosis model with or without the treatment of AhR activator 6-formylindolo(3,2-b)carbazole (FICZ). Positron emission tomography computed tomography (PET-CT) imaging, Periodic acid-Schiff (PAS) staining, and polarized light optical microscopy were used to evaluate kidney injury and crystal deposition in mice kidney. Western blotting, immunofluorescence, chromatin immunoprecipitation, microRNA-fluorescence in situ hybridization, and luciferase reporter assays were applied to analyze polarization state and regulation mechanism of macrophage. Results: AhR expression was significantly upregulated and negatively correlated with interferon-regulatory factor 1 (IRF1) and hypoxia inducible factor 1-alpha (HIF-1α) levels in a murine CaOx nephrocalcinosis model following administration of FICZ. Moreover, AhR activation suppressed IRF1 and HIF-1α levels and decreased M1 macrophage polarization in vitro. In terms of the mechanism, bioinformatics analysis and chromatin immunoprecipitation assay confirmed that AhR could bind to miR-142a promoter to transcriptionally activate miR-142a. In addition, luciferase reporter assays validated that miR-142a inhibited IRF1 and HIF-1α expression by directly targeting their 3'-untranslated regions. Conclusions: Our results indicated that AhR activation could diminish M1 macrophage polarization and promote M2 macrophage polarization to suppress CaOx nephrocalcinosis via the AhR-miR-142a-IRF1/HIF-1α pathway.
Keywords: AhR; HIF-1α; IRF1; Macrophage; Nephrocalcinosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Zeng G, Mai Z, Xia S, Wang Z, Zhang K, Wang L. et al. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int. 2017;120:109–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

