IL-33/ST2 induces neutrophil-dependent reactive oxygen species production and mediates gout pain
- PMID: 33204337
- PMCID: PMC7667675
- DOI: 10.7150/thno.48028
IL-33/ST2 induces neutrophil-dependent reactive oxygen species production and mediates gout pain
Abstract
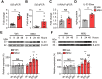
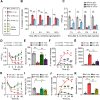
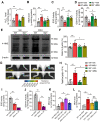
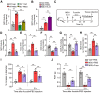
Objective: Gout, induced by monosodium urate (MSU) crystal deposition in joint tissues, provokes severe pain and impacts life quality of patients. However, the mechanisms underlying gout pain are still incompletely understood. Methods: We established a mouse gout model by intra-articularly injection of MSU crystals into the ankle joint of wild type and genetic knockout mice. RNA-Sequencing, in vivo molecular imaging, Ca2+ imaging, reactive oxygen species (ROS) generation, neutrophil influx and nocifensive behavioral assays, etc. were used. Results: We found interleukin-33 (IL-33) was among the top up-regulated cytokines in the inflamed ankle. Neutralizing or genetic deletion of IL-33 or its receptor ST2 (suppression of tumorigenicity) significantly ameliorated pain hypersensitivities and inflammation. Mechanistically, IL-33 was largely released from infiltrated macrophages in inflamed ankle upon MSU stimulation. IL-33 promoted neutrophil influx and triggered neutrophil-dependent ROS production via ST2 during gout, which in turn, activated transient receptor potential ankyrin 1 (TRPA1) channel in dorsal root ganglion (DRG) neurons and produced nociception. Further, TRPA1 channel activity was significantly enhanced in DRG neurons that innervate the inflamed ankle via ST2 dependent mechanism, which results in exaggerated nociceptive response to endogenous ROS products during gout. Conclusions: We demonstrated a previous unidentified role of IL-33/ST2 in mediating pain hypersensitivity and inflammation in a mouse gout model through promoting neutrophil-dependent ROS production and TRPA1 channel activation. Targeting IL-33/ST2 may represent a novel therapeutic approach to ameliorate gout pain and inflammation.
Keywords: Gout; TRPA1; arthritis; cytokine; neutrophil; reactive oxygen species.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Rees F, Hui M, Doherty M. Optimizing current treatment of gout. Nat Rev Rheumatol. 2014;10:271–83. - PubMed
-
- Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30–8. - PubMed
-
- Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda-Sanabria J. et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. - PubMed
-
- Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F. et al. Gout. Nat Rev Dis Primers. 2019;5:69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

