The Increasing Value of Biomass: Moving From C6 Carbohydrates to Multifunctionalized Building Blocks via 5-(hydroxymethyl)furfural
- PMID: 33204585
- PMCID: PMC7646257
- DOI: 10.1002/open.202000233
The Increasing Value of Biomass: Moving From C6 Carbohydrates to Multifunctionalized Building Blocks via 5-(hydroxymethyl)furfural
Abstract
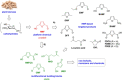
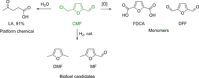
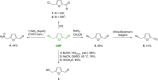
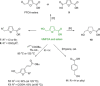
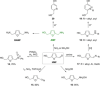
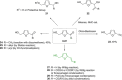
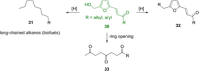
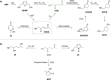
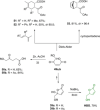
Recent decades have been marked by enormous progress in the field of synthesis and chemistry of 5-(hydroxymethyl)furfural (HMF), an important platform chemical widely recognized as the "sleeping giant" of sustainable chemistry. This multifunctional furanic compound is viewed as a strong link for the transition from the current fossil-based industry to a sustainable one. However, the low chemical stability of HMF significantly undermines its synthetic potential. A possible solution to this problem is synthetic diversification of HMF by modifying it into more stable multifunctional building blocks for further synthetic purposes.
Keywords: 5-(hydroxymethyl)furfural; biorefining; carbohydrates; plant biomass; renewable building blocks; sustainable chemistry.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- None
-
- De Clercq R., Dusselier M., Sels B. F., Green Chem. 2017, 19, 5012–5040;
-
- Clark J. H., Deswarte F. E. I., in Introduction to Chemicals from Biomass (Eds.: Clark J. H., Deswarte F. E. I.), Wiley, Chichester, 2008, pp. 1–20.
-
- Palkovits R., Wright W. R. H., in Chemical Energy Storage (Ed.: Schlögl R.), De Gruyter, Berlin, 2012, pp. 59–86.
-
- See, for example:
Publication types
LinkOut - more resources
Full Text Sources

