Cellular and molecular outcomes of glutamine supplementation in the brain of succinic semialdehyde dehydrogenase-deficient mice
- PMID: 33204597
- PMCID: PMC7653255
- DOI: 10.1002/jmd2.12151
Cellular and molecular outcomes of glutamine supplementation in the brain of succinic semialdehyde dehydrogenase-deficient mice
Abstract
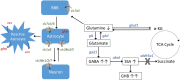
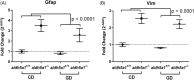
Succinic semialdehyde dehydrogenase deficiency (SSADHD) manifests with low levels of glutamine in the brain, suggesting that central glutamine deficiency contributes to pathogenesis. Recently, we attempted to rescue the disease phenotype of aldh5a1 -/- mice, a murine model of SSADHD with dietary glutamine supplementation. No clinical rescue and no central glutamine improvement were observed. Here, we report the results of follow-up studies of the cellular and molecular basis of the resistance of the brain to glutamine supplementation. We first determined if the expression of genes involved in glutamine metabolism was impacted by glutamine feeding. We then searched for changes of brain histology in response to glutamine supplementation, with a focus on astrocytes, known regulators of glutamine synthesis in the brain. Glutamine supplementation significantly modified the expression of glutaminase (gls) (0.6-fold down), glutamine synthetase (glul) (1.5-fold up), and glutamine transporters (solute carrier family 7, member 5 [slc7a5], 2.5-fold up; slc38a2, 0.6-fold down). The number of GLUL-labeled cells was greater in the glutamine-supplemented group than in controls (P < .05). Reactive astrogliosis, a hallmark of brain inflammation in SSADHD, was confirmed. We observed a 2-fold stronger astrocyte staining in mutants than in wild-type controls (optical density/cell were 1.8 ± 0.08 in aldh5a1 -/- and 0.99 ± 0.06 in aldh5a1 +/+ ; P < .0001), and a 3-fold higher expression of gfap and vimentin. However, glutamine supplementation did not improve the histological and molecular signature of astrogliosis. Thus, glutamine supplementation impacts genes implicated in central glutamine homeostasis without improving reactive astrogliosis. The mechanisms underlying glutamine deficiency and its contribution to SSADHD pathogenesis remain unknown and should be the focus of future investigations.
Keywords: GABA; GHB; astrocyte; dietary supplementation; glutamine; knockout mice.
© 2020 The Authors. JIMD Reports published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures



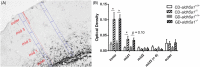
References
-
- Shank RP, Aprison MH. Glutamine uptake and metabolism by the isolated toad brain: evidence pertaining to its proposed role as a transmitter precursor. J Neurochem. 1977;28(6):1189‐1196. - PubMed
-
- Tapia R, Gonzalez RM. Glutamine and glutamate as precursors of the releasable pool of gaba in brain cortex slices. Neurosci Lett. 1978;10(1‐2):165‐169. - PubMed
-
- Reubi JC, Van Der Berg C, Cuenod M. Glutamine as precursor for the GABA and glutamate trasmitter pools. Neurosci Lett. 1978;10(1‐2):171‐174. - PubMed
-
- Gibson KM, Gupta M, Pearl PL, et al. Significant behavioral disturbances in succinic semialdehyde dehydrogenase (SSADH) deficiency (gamma‐hydroxybutyric aciduria). Biol Psychiatry. 2003;54(7):763‐768. - PubMed
-
- Gupta M, Polinsky M, Senephansiri H, et al. Seizure evolution and amino acid imbalances in murine succinate semialdehyde dehydrogenase (SSADH) deficiency. Neurobiol Dis. 2004;16(3):556‐562. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

