An adapted novel flow cytometry methodology to delineate types of cell death in airway epithelial cells
- PMID: 33204742
- PMCID: PMC7666329
- DOI: 10.14440/jbm.2020.336
An adapted novel flow cytometry methodology to delineate types of cell death in airway epithelial cells
Abstract
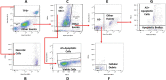
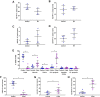
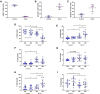
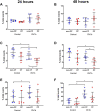
Current methodologies to measure apoptotic and necrotic cell death using flow cytometry do not adequately differentiate between the two. Here, we describe a flow cytometry methodology adapted to airway epithelial cells (AEC) to sufficiently differentiate apoptotic and necrotic AEC. Specifically, cell lines and primary AEC (n = 12) were permeabilized or infected with rhinovirus 1b (RV1b) over 48 h. Cell death was then measured via annexin V/propidium iodide (A5/PI) or annexin V/TO-PRO-3 (A5/TP3) staining using a novel flow cytometry and gating methodology adapted to AEC. We show that A5/PI staining could not sufficiently differentiate between types of cell death following RV1b infection of primary AEC. However, A5/TP3 staining was able to distinguish six cell death populations (viable, necrotic, debris, A5+ apoptotic, A5- apoptotic, apoptotic bodies) after permeabilization or infection with RV1b, with phenotypic differences were observed in apoptotic populations. Collectively, using a staining and gating strategy never adapted to AEC, A5/TP3 could accurately differentiate and quantify viable, necrotic, and apoptotic AEC following RV1b infection.
Keywords: airway epithelium; cell death; flow cytometry; rhinovirus.
© 2013-2020 The Journal of Biological Methods, All rights reserved.
Conflict of interest statement
Competing interests: The authors have declared that no competing interests exist.
Figures
References
-
- Gern JE, Dick EC, Lee WM, Murray S, Meyer K, et al. (1996) Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J Immunol 156: 621-627. PMID: - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
