Comparison of outcomes following transfemoral versus trans-subclavian approach for transcatheter aortic valve Implantation: A meta-analysis
- PMID: 33204819
- PMCID: PMC7653062
- DOI: 10.1016/j.ijcha.2020.100668
Comparison of outcomes following transfemoral versus trans-subclavian approach for transcatheter aortic valve Implantation: A meta-analysis
Abstract
Background: The subclavian artery is an alternative access route for transcatheter aortic valve implantation (TAVI), with a potential advantage in patients unsuitable for traditional access routes such as the femoral artery. This study aimed to determine the safety and efficacy of the trans-subclavian (TSc) compared to the trans-femoral (TF) approach.
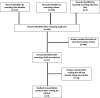
Methods: A systematic review was conducted on two online databases: Embase and Medline. The initial search returned 508 titles. Nine observational studies were included: n = 2938 patients (2382 TF and 556 TSc).
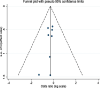
Results: Both TSc and TF groups were comparable for: 30-day mortality (Odds ratio, OR 0.75, 95% CI 0.49 - 1.16, p = 0.195); in-hospital stroke (OR 1.05, 95% CI 0.60-1.85, p = 0.859); myocardial infarction (OR 1.97, 95% CI 0.74-5.23, p = 0.176); paravalvular leaks (OR 1.20, 95% CI 0.76-1.90, p = 0.439); rates of postoperative permanent pacemaker implantation (OR 1.49, 95% CI 0.92-2.41, p = 0.105); in-hospital bleeding and meta-analysis demonstrated no significant difference between access points (OR 3.44, 95% CI 0.35-34.22, p = 0.292). Procedural time was found to be longer in the TSc group (SMD 1.02; 95% CI 0.815-1.219, p < 0.001). Major vascular complications were significantly higher in the TF group (OR 0.55, 95% CI 0.32-0.94, p = 0.029). Meta regression found no influence of the covariates on the outcomes.
Conclusion: Subclavian access is both a safe and feasible alternative access route for TAVI with lower risks of major vascular complications. This study supports the use of subclavian access as a viable alternative in patient groups where transfemoral TAVI is contraindicated.
Keywords: Femoral artery; Subclavian artery; Transcatheter aortic valve; Vascular complications.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Cribier A., Eltchaninoff H., Tron C., Bauer F., Derumeaux G., Bash A., Borenstein N. Percutaneous implantation of prosthetic heart valves: From animal model to first human implantation in calcific aortic stenosis. J. Am. Coll. Cardiol. 2003;41(6):508. doi: 10.1016/S0735-1097(03)82753-5. - DOI - PubMed
-
- Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., III, Fleisher L.A., Jneid H., Mack M.J., McLeod C.J., O’Gara P.T., Rigolin V.H., Sundt T.M., III, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. J. Am. Coll. Cardiol. 2017;70(2):252–289. - PubMed
-
- Hayashida K., Lefvre T., Chevalier B., Hovasse T., Romano M., Garot P. Transfemoral aortic valve implantation: New criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4(8):851–858. - PubMed
-
- Overtchouk P., Modine T. A comparison of alternative access routes for transcatheter aortic valve implantation. Expert Rev Cardiovasc Ther. 2018;16(10):749–756. - PubMed
-
- Mack M.J., Leon M.B., Smith C.R., Miller D.C., Moses J.W., Tuzcu E.M. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet. 2015;385(9986):2477–2484. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

