Mitofusin-2 mediates doxorubicin sensitivity and acute resistance in Jurkat leukemia cells
- PMID: 33204855
- PMCID: PMC7648112
- DOI: 10.1016/j.bbrep.2020.100824
Mitofusin-2 mediates doxorubicin sensitivity and acute resistance in Jurkat leukemia cells
Abstract
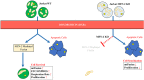
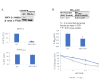
Mitochondria oscillate along a morphological continuum from fragmented individual units to hyperfused tubular networks. Their position at the junction of catabolic and anabolic metabolism couples this morphological plasticity, called mitochondrial dynamics, to larger cellular metabolic programs, which in turn implicate mitochondria in a number of disease states. In many cancers, fragmented mitochondria engage the cell with the biosynthetic capacity of aerobic glycolysis in service of proliferation and progression. Chemo-resistant cancers, however, favor remodeling dynamics that yield fused mitochondrial assemblies utilizing oxidative phosphorylation (OXPHOS) through the electron transport chain (ETC). In this study, expression of Mitofusin-2 (MFN-2), a GTPase protein mediator of mitochondrial fusion, was found to closely correlate to Jurkat leukemia cell survival post doxorubicin (DxR) assault. Moreover, this was accompanied by dramatically increased expression of OXPHOS respiratory complexes and ATP Synthase, as well as a commensurate escalation of state III respiration and respiratory control ratio (RCR). Importantly, CRISPR knockout of MFN-2 resulted in a considerable decrease of doxorubicin (DxR) median lethal dose compared to a treated wildtype control, suggesting an important role of mitochondrial fusion in chemotherapy sensitivity and acute resistance.
Keywords: Doxorubicin; MFN-2; Mitochondrial fusion; OXPHOS; Sensitivity.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Vos T., Allen C., Arora M., Barber R.M., Bhutta Z.A., Brown A., Carter A., Casey D.C., Charlson F.J., Chen A.Z., Coggeshall M., Cornaby L., Dandona L., Dicker D.J., Dilegge T., Erskine H.E., Ferrari A.J., Fitzmaurice C., Fleming T.…Murray C.J.L. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/s0140-6736(16)31678-6. - DOI - PMC - PubMed
-
- Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A., Casey D.C., Charlson F.J., Chen A.Z., Coates M.M., Coggeshall M., Dandona L., Dicker D.J., Erskine H.E., Ferrari A.J., Fitzmaurice C., Foreman K., Forouzanfar M.H., Fraser M.S.…Murray C.J.L. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/s0140-6736(16)31012-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

