Transgenic goats producing an improved version of cetuximab in milk
- PMID: 33205005
- PMCID: PMC7655094
- DOI: 10.1096/fba.2020-00059
Transgenic goats producing an improved version of cetuximab in milk
Abstract
Therapeutic monoclonal antibodies (mAbs) represent one of the most important classes of pharmaceutical proteins to treat human diseases. Most are produced in cultured mammalian cells which is expensive, limiting their availability. Goats, striking a good balance between a relatively short generation time and copious milk yield, present an alternative platform for the cost-effective, flexible, large-scale production of therapeutic mAbs. Here, we focused on cetuximab, a mAb against epidermal growth factor receptor, that is commercially produced under the brand name Erbitux and approved for anti-cancer treatments. We generated several transgenic goat lines that produce cetuximab in their milk. Two lines were selected for detailed characterization. Both showed stable genotypes and cetuximab production levels of up to 10 g/L. The mAb could be readily purified and showed improved characteristics compared to Erbitux. The goat-produced cetuximab (gCetuximab) lacked a highly immunogenic epitope that is part of Erbitux. Moreover, it showed enhanced binding to CD16 and increased antibody-dependent cell-dependent cytotoxicity compared to Erbitux. This indicates that these goats produce an improved cetuximab version with the potential for enhanced effectiveness and better safety profile compared to treatments with Erbitux. In addition, our study validates transgenic goats as an excellent platform for large-scale production of therapeutic mAbs.
Keywords: EGFR; biobetter; biosimilar; cetuximab; monoclonal antibody.
© 2020 The Authors. FASEB BioAdvances published by the Federation of American Societies for Experimental Biology.
Conflict of interest statement
GL, SC, BB, PM, and DNW are employees of AgResearch, and LHC, DPP, NCM, WGG, HMM, NF, and CDR are employees of LFB‐USA and LFB Biotechnologies, respectively. All these organizations have a commercial interests or potential commercial interests in the production of gCetuximab. LC has no conflict of interest or financial conflict to disclose.
Figures



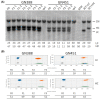

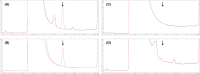

References
-
- Walsh G. Biopharmaceutical benchmarks 2014. Nat Biotechnol. 2014;32:992‐1000. - PubMed
-
- Durocher Y, Butler M. Expression systems for therapeutic glycoprotein production. Curr Opin Biotechnol. 2009;20:700‐707. - PubMed
-
- Hacker DL, De Jesus M, Wurm FM. 25 years of recombinant proteins from reactor‐grown cells ‐ where do we go from here? Biotechnol Adv. 2009;27:1023‐1027. - PubMed
-
- Dyck MK, Lacroix D, Pothier F, Sirard MA. Making recombinant proteins in animals–different systems, different applications. Trends Biotechnol. 2003;21:394‐399. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
