Long-term outcomes in patients with PET-predicted poor-responsive HER2-positive breast cancer treated with neoadjuvant bevacizumab added to trastuzumab and docetaxel: 5-year follow-up of the randomised Avataxher study
- PMID: 33205032
- PMCID: PMC7649610
- DOI: 10.1016/j.eclinm.2020.100566
Long-term outcomes in patients with PET-predicted poor-responsive HER2-positive breast cancer treated with neoadjuvant bevacizumab added to trastuzumab and docetaxel: 5-year follow-up of the randomised Avataxher study
Abstract
Background: The open-label, randomised Phase 2 AVATAXHER study (NCT01142778) demonstrated that early PET assessment identified HER2-positive breast cancer patients who responded poorly to neoadjuvant docetaxel plus trastuzumab. Adding neoadjuvant bevacizumab for PET-predicted poor-responders improved pathological complete response (pCR) rates (43.8% vs 24.0%). We investigated long-term study outcomes.
Methods: Patients were treated in three groups. All patients initially received two cycles of standard neoadjuvant therapy with [¹⁸F]-FDG PET conducted before each cycle. Those with ≥70% change in the maximum standardised uptake value (∆SUVmax) received four further cycles of standard neoadjuvant therapy (PET responders). PET-predicted poor-responders (∆SUVmax <70%) were randomised (2:1) to neoadjuvant therapy with (Group A) or without (Group B) bevacizumab for cycles 3-6. All patients received one further cycle of trastuzumab before surgery plus adjuvant trastuzumab (11 cycles).
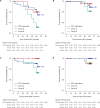
Findings: 142 patients were randomized and treated (PET responders, n = 69; Group A, n = 48; Group B, n = 25). 5-year disease-free survival rates were 90.5% (95% CI: 80.0-95.6%) in PET responders, 90.2% (95% CI: 75.9-96.2%) in Group A, and 76.0% (95% CI: 54.2-88.4%) in Group B. However, no difference was observed between randomised arms in a sensitivity analysis. During adjuvant therapy, the incidence of Grade ≥3 (Group A: 25.6%; Group B 12.5%) and serious adverse events (Group A: 18.6%; Group B 12.5%) was higher in Group A vs Group B, but with no apparent effect on cardiac events.
Interpretation: In patients with HER2-positive breast cancer, an intervention based on early PET assessment and improvement of pCR does not modify disease-free survival.
Funding: Roche France.
Keywords: Bevacizumab; Early pet assessment; Her-2 positive breast cancer; Long-term follow-up; Neoadjuvant; Positron emission tomography; pathological complete response; Δsuvmax.
© 2020 The Authors.
Conflict of interest statement
BC reports personal fees and non-financial support from Roche Laboratories France, personal fees from Bristol Myers Laboratories France, and personal fees from AstraZeneca Laboratories. JYP declares research funding received by his institution from Roche and Menarini Silicon Biosystems; honoraria received by his institution from Roche and Sanofi; and personal fees from Roche, Novartis, AstraZeneca, Pfizer, Amgen, Ipsen, Lilly, and Genomic Health. TP is a member of scientific boards for Novartis, Lilly and Pfizer. TB reports personal fees and non-financial support from Roche, Novartis, AstraZeneca, and Pfizer; and personal fees from Seattle Genetics, outside the submitted work. DC reports honorarium from Roche, Pfizer, Novartis, Sanofi, and AstraZeneca. GP acted as consultant for Chugai, Novartis and Shire (Takeda), with remunerations received by his institution. GP's research team received grants for academic studies from Amgen, Genzyme (Sanofi), Lilly, Merck, Novartis, and Roche Pharma. AF and JD are full-time employees of Roche Pharma France. KK, J-MF, and PG report personal fees from Roche during the conduct of the study. LA reports personal fees and non-financial support from Roche Laboratories France and Bristol Myers Laboratories France, and personal fees from AstraZeneca Laboratories and Merck. M-AM-R, FLD, P-FD, M-PC, CB, J-BP, GT, and AB-R declare no conflicts of interest.
Figures

References
-
- Buzdar A.U., Ibrahim N.K., Francis D. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. - PubMed
-
- Gianni L., Pienkowski T., Im Y.H. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. - PubMed
-
- Humbert O., Riedinger J.M., Vrigneaud J.M. 18F-FDG PET-derived tumor blood flow changes after 1 cycle of neoadjuvant chemotherapy predicts outcome in triple-negative breast cancer. J Nucl Med. 2016;57:1707–1712. - PubMed
-
- Garcia Garcia-Esquinas M.A., Arrazola G.J., Garcia-Saenz J.A. Predictive value of PET-CT for pathological response in stages II and III breast cancer patients following neoadjuvant chemotherapy with docetaxel. Rev Esp Med Nucl Imagen Mol. 2014;33:14–21. - PubMed
-
- Gebhart G., Gamez C., Holmes E. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO. J Nucl Med. 2013;54:1862–1868. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

