Elevated Serum Amino Acids Induce a Subpopulation of Alpha Cells to Initiate Pancreatic Neuroendocrine Tumor Formation
- PMID: 33205067
- PMCID: PMC7659536
- DOI: 10.1016/j.xcrm.2020.100058
Elevated Serum Amino Acids Induce a Subpopulation of Alpha Cells to Initiate Pancreatic Neuroendocrine Tumor Formation
Abstract
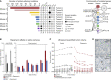
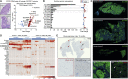
The cellular origin of sporadic pancreatic neuroendocrine tumors (PNETs) is obscure. Hormone expression suggests that these tumors arise from glucagon-producing alpha cells or insulin-producing β cells, but instability in hormone expression prevents linage determination. We utilize loss of hepatic glucagon receptor (GCGR) signaling to drive alpha cell hyperproliferation and tumor formation to identify a cell of origin and dissect mechanisms that drive progression. Using a combination of genetically engineered Gcgr knockout mice and GCGR-inhibiting antibodies, we show that elevated plasma amino acids drive the appearance of a proliferative population of SLC38A5+ embryonic progenitor-like alpha cells in mice. Further, we characterize tumors from patients with rare bi-allelic germline GCGR loss-of-function variants and find prominent tumor-cell-associated expression of the SLC38A5 paralog SLC7A8 as well as markers of active mTOR signaling. Thus, progenitor cells arise from adult alpha cells in response to metabolic signals and, when inductive signals are chronically present, drive tumor initiation.
Keywords: GCGR; Glucagon; PNET; SLC38A5; SLC38A7; mTOR; pancreatic neuroendocrine tumor; serum amino-acids; tumorigenesis.
© 2020 The Authors.
Figures



References
-
- Bajetta E., Procopio G., Ferrari L., Catena L., Del Vecchio M., Bombardieri E. Update on the treatment of neuroendocrine tumors. Expert Rev. Anticancer Ther. 2003;3:631–642. - PubMed
-
- Sharma N., Naraev B.G., Engelman E.G., Zimmerman M.B., Bushnell D.L., Jr., OʼDorisio T.M., OʼDorisio M.S., Menda Y., Müller-Brand J., Howe J.R., Halfdanarson T.R. Peptide receptor radionuclide therapy outcomes in a North American cohort with metastatic well-differentiated neuroendocrine tumors. Pancreas. 2017;46:151–156. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

