Macrophage depletion of CMV latently infected donor hearts ameliorates recipient accelerated chronic rejection
- PMID: 33205500
- PMCID: PMC8068575
- DOI: 10.1111/tid.13514
Macrophage depletion of CMV latently infected donor hearts ameliorates recipient accelerated chronic rejection
Abstract
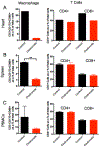
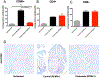
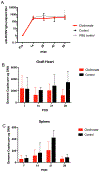
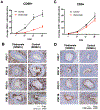
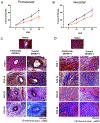
Cytomegalovirus (CMV) infection is linked to acceleration of solid organ transplant vascular sclerosis (TVS) and chronic rejection (CR). Donor latent CMV infection increases cardiac-resident macrophages and T cells leading to increased inflammation, promoting allograft rejection. To investigate the role of cardiac-resident passenger macrophages in CMV-mediated TVS/CR, macrophages were depleted from latently ratCMV (RCMV)-infected donor allografts prior to transplantation. Latently RCMV-infected donor F344 rats were treated with clodronate, PBS, or control liposomes 3 days prior to cardiac transplant into RCMV-naïve Lewis recipients. Clodronate treatment significantly increased graft survival from post-operative day (POD)61 to POD84 and decreased TVS at rejection. To determine the kinetics of the effect of clodronate treatment's effect, a time study revealed that clodronate treatment significantly decreased macrophage infiltration into allograft tissues as early as POD14; altered allograft cytokine/chemokine protein levels, fibrosis development, and inflammatory gene expression profiles. These findings support our hypothesis that increased graft survival as a result of allograft passenger macrophage depletion was in part a result of altered immune response kinetics. Depletion of donor macrophages prior to transplant is a strategy to modulate allograft rejection and reduce TVS in the setting of CMV + donors transplanted into CMV naïve recipients.
Keywords: chronic rejection; cytomegalovirus; latent infection; transplant vascular sclerosis.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
Conflict of Interest
The authors do not have any conflicts of interest.
Figures





References
-
- Hodson EM, Barclay PG, Craig JC, et al. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2005(4):Cd003774. - PubMed
-
- Merigan TC, Renlund DG, Keay S, et al. A controlled trial of ganciclovir to prevent cytomegalovirus disease after heart transplantation. N Engl J Med. 1992;326(18):1182–1186. - PubMed
-
- Valentine VG, Weill D, Gupta MR, et al. Ganciclovir for cytomegalovirus: a call for indefinite prophylaxis in lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2008;27(8):875–881. - PubMed

