Etanercept or Methotrexate Withdrawal in Rheumatoid Arthritis Patients in Sustained Remission
- PMID: 33205906
- PMCID: PMC8251940
- DOI: 10.1002/art.41589
Etanercept or Methotrexate Withdrawal in Rheumatoid Arthritis Patients in Sustained Remission
Abstract
Objective: Patients with rheumatoid arthritis (RA) in whom remission is achieved following combination therapy with methotrexate plus etanercept face an ongoing medication burden. This study was undertaken to investigate whether sustained remission achieved on combination therapy can be maintained with either methotrexate or etanercept monotherapy, as assessed following discontinuation of one or the other medication from the combination.
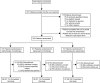
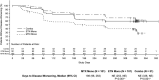
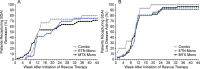
Methods: Of the 371 adult patients with RA who received combination therapy with methotrexate plus etanercept, remission (defined as a Simplified Disease Activity Index [SDAI] score of ≤3.3) was sustained in 253 patients through a 24-week open-label period. These 253 patients then entered a 48-week, double-blind period and were randomized to receive either 1) methotrexate monotherapy (n = 101), 2) etanercept monotherapy (n = 101), or 3) methotrexate plus etanercept combination therapy (n = 51). Patients who subsequently experienced disease-worsening received rescue therapy with the combination regimen at the same dosages as used in the initial run-in period. The primary end point was the proportion of patients in whom SDAI-defined remission was maintained without disease-worsening at week 48 in the etanercept monotherapy group as compared to the methotrexate monotherapy group. Secondary end points included time to disease-worsening, and the proportion of patients in whom SDAI-defined remission was recaptured after initiation of rescue therapy.
Results: Baseline demographic and clinical characteristics of the RA patients were similar across the treatment groups. At week 48, SDAI-defined remission was maintained in significantly more patients in the etanercept monotherapy group than in the methotrexate monotherapy group (49.5% versus 28.7%; P = 0.004). Moreover, as a secondary end point, sustained SDAI-defined remission was achieved in significantly more patients who received combination therapy than in those who received methotrexate monotherapy (52.9% versus 28.7%; P = 0.006). Time to disease-worsening was shorter in those who received methotrexate monotherapy than in those who received etanercept monotherapy or those who received combination therapy (each P < 0.001 versus methotrexate monotherapy). Among the patients who received rescue therapy, SDAI-defined remission was recaptured in 70-80% in each treatment group. No new safety signals were reported.
Conclusion: The efficacy of etanercept monotherapy was superior to that of methotrexate monotherapy and similar to that of combination therapy in maintaining remission in patients with RA. SDAI-defined remission was recaptured in most of the patients who were given rescue therapy. These data could inform decision-making when withdrawal of therapy is being considered to reduce treatment burden in patients with well-controlled RA.
Trial registration: ClinicalTrials.gov NCT02373813.
© 2020 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

Comment in
-
Reply.Arthritis Rheumatol. 2021 Sep;73(9):1771-1772. doi: 10.1002/art.41718. Epub 2021 Aug 9. Arthritis Rheumatol. 2021. PMID: 33750019 No abstract available.
-
Etanercept or Methotrexate Withdrawal in Rheumatoid Arthritis Patients Receiving Combination Therapy: Comment on the Article by Curtis et al.Arthritis Rheumatol. 2021 Sep;73(9):1771. doi: 10.1002/art.41719. Epub 2021 Aug 9. Arthritis Rheumatol. 2021. PMID: 33750021 No abstract available.
References
-
- Hamann P, Holland R, Hyrich K, Pauling JD, Shaddick G, Nightingale A, et al. Factors associated with sustained remission in rheumatoid arthritis in patients treated with anti–tumor necrosis factor. Arthritis Care Res (Hoboken) 2017;69:783–93. - PubMed
-
- Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018;320:1360–72. - PubMed
-
- Edwards CJ, Galeazzi M, Bellinvia S, Ringer A, Dimitroulas T, Kitas G. Can we wean patients with inflammatory arthritis from biological therapies? [review]. Autoimmun Rev 2019;18:102399. - PubMed
-
- Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double‐blind randomised controlled trial. Lancet 2004;363:675–81. - PubMed
-
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double‐blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

