Efficacy and Safety of Inhaled Ciclesonide in Treating Patients With Asymptomatic or Mild COVID-19 in the RACCO Trial: Protocol for a Multicenter, Open-label, Randomized Controlled Trial
- PMID: 33206053
- PMCID: PMC7781585
- DOI: 10.2196/23830
Efficacy and Safety of Inhaled Ciclesonide in Treating Patients With Asymptomatic or Mild COVID-19 in the RACCO Trial: Protocol for a Multicenter, Open-label, Randomized Controlled Trial
Abstract
Background: Currently, there are no specific effective treatments for SARS-CoV-2 infection; however, various COVID-19 treatment options are under investigation. It is vital to continue investigating the landscape of SARS-CoV-2-induced pneumonia and therapeutic interventions.
Objective: This paper presents the protocol for a randomized controlled trial that aims to compare the pneumonia exacerbation rate between ciclesonide (ALVESCO; Teijin Pharma Limited) administration and symptomatic treatment in patients with COVID-19 and to determine the efficacy of ciclesonide. The secondary objectives are to investigate the safety of ciclesonide administration, changes in clinical and laboratory findings, and the number of viral genome copies of SARS-CoV-2 over time between the 2 groups.
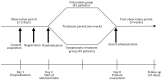
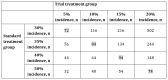
Methods: In this investigator-initiated, exploratory, prospective, multicenter, parallel-group, open-label, randomized controlled trial, a total of 90 patients diagnosed with COVID-19 will be recruited from 21 hospitals in Japan based on specific inclusion and exclusion criteria. Participants will be randomized either to the ciclesonide group, which will receive a 400-µg dose of ciclesonide 3 times per day over a 7-day period, or to the symptomatic treatment group. Both groups will receive antitussives and antipyretics as required. Data collection for various parameters will be conducted on days 1, 2, 4, 8, 22, and 29 to record baseline assessments and the findings over an extended period. Computed tomography images taken prior to drug administration and 1 week following treatment will be compared, and efficacy will be confirmed by checking for pneumonia exacerbation. Primary endpoint analysis will be performed using the Fisher exact test to determine statistically significant differences in the pneumonia exacerbation rate between the ciclesonide and symptomatic treatment groups.
Results: The first trial participant was enrolled on April 3, 2020. Recruitment is expected to be completed on September 30, 2020, while follow-up assessments of all participants are expected to be completed by October 31, 2020. The study results will be published in a peer-reviewed scientific journal.
Conclusions: The RACCO (Randomized Ciclesonid COVID-19) study will provide definitive comparative effectiveness data and important clinical outcomes data between the ciclesonide and symptomatic treatment groups. If the hypotheses that pneumonia exacerbation rate reduction is more significant in the ciclesonide treatment group than in the symptomatic treatment group and that ciclesonide is safe for use are valid, ciclesonide will serve as an important therapeutic option for patients with COVID-19.
Trial registration: Japan Registry of Clinical Trials jRCTs031190269; https://jrct.niph.go.jp/en-latest-detail/jRCTs031190269.
International registered report identifier (irrid): DERR1-10.2196/23830.
Keywords: COVID-19; SARS-CoV-2; drug; intervention; proposed therapy; protocol; randomized controlled trial; therapy; treatment.
©Junko Terada-Hirashima, Manabu Suzuki, Yukari Uemura, Masayuki Hojo, Ayako Mikami, Wataru Sugiura, Norio Ohmagari, Haruhito Sugiyama. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 31.12.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020 Feb 15;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(20)30185-9 - DOI - PMC - PubMed
-
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. http://europepmc.org/abstract/MED/32087114 - DOI - PMC - PubMed
-
- Archived: WHO Timeline - COVID-19. World Health Organization. 2020. Apr 27, [2020-12-03]. https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19.
-
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. http://europepmc.org/abstract/MED/32109013 - DOI - PMC - PubMed
-
- Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, Sun W, Wang Y. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Jun;92(6):577–583. doi: 10.1002/jmv.25757. http://europepmc.org/abstract/MED/32162702 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

