Silencing the spindle assembly checkpoint: Let's play Polo!
- PMID: 33206134
- PMCID: PMC7716382
- DOI: 10.1083/jcb.202010053
Silencing the spindle assembly checkpoint: Let's play Polo!
Abstract
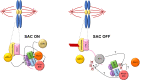
Silencing of the spindle assembly checkpoint involves two protein phosphatases, PP1 and PP2A-B56, that are thought to extinguish checkpoint signaling through dephosphorylation of a checkpoint scaffold at kinetochores. In this issue, Cordeiro et al. (2020. J. Cell Biol.https://doi.org/10.1083/jcb.202002020) now show that a critical function of these phosphatases in checkpoint silencing is removal of Polo kinase at kinetochores, which would otherwise autonomously sustain the checkpoint.
© 2020 Benzi and Piatti.
Figures
Comment on
-
Kinetochore phosphatases suppress autonomous Polo-like kinase 1 activity to control the mitotic checkpoint.J Cell Biol. 2020 Dec 7;219(12):e202002020. doi: 10.1083/jcb.202002020. J Cell Biol. 2020. PMID: 33125045 Free PMC article.
References
-
- Musacchio, A. 2015. Curr. Biol. 10.1016/j.cub.2015.08.051 - DOI

