Association of Race/Ethnicity-Specific Changes in Antihypertensive Medication Classes Initiated Among Medicare Beneficiaries With the Eighth Joint National Committee Panel Member Report
- PMID: 33206191
- PMCID: PMC7675104
- DOI: 10.1001/jamanetworkopen.2020.25127
Association of Race/Ethnicity-Specific Changes in Antihypertensive Medication Classes Initiated Among Medicare Beneficiaries With the Eighth Joint National Committee Panel Member Report
Abstract
Importance: In December 2013, the panel members appointed to the Eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC8) published a recommendation that non-Black adults initiate antihypertensive medication with a thiazide-type diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor (ACEI), or angiotensin receptor blocker (ARB), whereas Black adults initiate treatment with a thiazide-type diuretic or calcium channel blocker. β-Blockers were not recommended as first-line therapy.
Objective: To assess changes in antihypertensive medication classes initiated by race/ethnicity from before to after publication of the JNC8 panel member report.
Design, setting, and participants: This serial cross-sectional analysis assessed a 5% sample of Medicare beneficiaries aged 66 years or older who initiated antihypertensive medication between 2011 and 2018, were Black (n = 3303 [8.0%]), White (n = 34 943 [84.5%]), or of other (n = 3094 [7.5%]) race/ethnicity, and did not have compelling indications for specific antihypertensive medication classes.
Exposures: Calendar year and period after vs before publication of the JNC8 panel member report.
Main outcomes and measures: The proportion of beneficiaries initiating ACEIs or ARBs and, separately, β-blockers vs other antihypertensive medication classes.
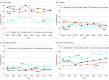
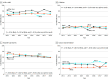
Results: In total, 41 340 Medicare beneficiaries (65% women; mean [SD] age, 75.7 [7.6] years) of Black, White, or other races/ethnicities initiated antihypertensive medication and met the inclusion criteria for the present study. In 2011, 25.2% of Black beneficiaries initiating antihypertensive monotherapy did so with an ACEI or ARB compared with 23.7% in 2018 (P = .47 for trend). Among beneficiaries initiating monotherapy, the proportion filling a β-blocker was 20.1% in 2011 and 15.4% in 2018 for White beneficiaries (P < .001 for trend), 14.2% in 2011 and 11.1% in 2018 for Black beneficiaries (P = .08 for trend), and 11.3% in 2011 and 15.0% in 2018 for beneficiaries of other race/ethnicity (P = .40 for trend). After multivariable adjustment and among beneficiaries initiating monotherapy, there was no evidence of a change in the proportion filling an ACEI or ARB before to after publication of the JNC8 panel member report overall (prevalence ratio, 1.00; 95% CI, 0.97-1.03) or in Black vs White beneficiaries (prevalence ratio, 0.96; 95% CI, 0.83-1.12; P = .60 for interaction). Among beneficiaries initiating monotherapy, the proportion filling a β-blocker decreased from before to after publication of the JNC8 panel member report (prevalence ratio, 0.89; 95% CI, 0.84-0.93) with no differences across race/ethnicity groups (P > .10 for interaction).
Conclusions and relevance: A substantial proportion of older US adults who initiate antihypertensive medication do so with non-guideline-recommended classes of medication.
Conflict of interest statement
Figures
Similar articles
-
Antihypertensive medication classes used among medicare beneficiaries initiating treatment in 2007-2010.PLoS One. 2014 Aug 25;9(8):e105888. doi: 10.1371/journal.pone.0105888. eCollection 2014. PLoS One. 2014. PMID: 25153199 Free PMC article.
-
Renin-Angiotensin-Aldosterone System-based Antihypertensive Agents and the Risk of Colorectal Cancer Among Medicare Beneficiaries.Epidemiology. 2019 Nov;30(6):867-875. doi: 10.1097/EDE.0000000000001065. Epidemiology. 2019. PMID: 31348009 Free PMC article.
-
National Trends in Antihypertensive Treatment Among Older Adults by Race and Presence of Comorbidity, 2008 to 2017.J Gen Intern Med. 2022 Dec;37(16):4223-4232. doi: 10.1007/s11606-022-07612-3. Epub 2022 Apr 26. J Gen Intern Med. 2022. PMID: 35474502 Free PMC article.
-
A Review of ACE Inhibitors and ARBs in Black Patients With Hypertension.Ann Pharmacother. 2018 Nov;52(11):1143-1151. doi: 10.1177/1060028018779082. Epub 2018 May 29. Ann Pharmacother. 2018. PMID: 29808707 Review.
-
Treating hypertension in Black patients.JAAPA. 2022 Feb 1;35(2):15-18. doi: 10.1097/01.JAA.0000791512.37549.64. JAAPA. 2022. PMID: 35076435 Review.
Cited by
-
Trends in Sudden Cardiac Death Related Mortality in Adults in the United States: A CDC WONDER Database Analysis, 1999-2020.Clin Cardiol. 2025 Jul;48(7):e70180. doi: 10.1002/clc.70180. Clin Cardiol. 2025. PMID: 40662462 Free PMC article.
-
Inequities in Hypertension Control in the United States Exposed and Exacerbated by COVID-19 and the Role of Home Blood Pressure and Virtual Health Care During and After the COVID-19 Pandemic.J Am Heart Assoc. 2021 Jun;10(11):e020997. doi: 10.1161/JAHA.121.020997. Epub 2021 May 19. J Am Heart Assoc. 2021. PMID: 34006116 Free PMC article.
-
Racial and Ethnic Differences in Blood Pressure Among US Adults, 1999-2018.Hypertension. 2021 Dec;78(6):1730-1741. doi: 10.1161/HYPERTENSIONAHA.121.18086. Epub 2021 Nov 1. Hypertension. 2021. PMID: 34719937 Free PMC article.
-
Tracking Blood Pressure Control Performance and Process Metrics in 25 US Health Systems: The PCORnet Blood Pressure Control Laboratory.J Am Heart Assoc. 2021 Nov 2;10(21):e022224. doi: 10.1161/JAHA.121.022224. Epub 2021 Oct 6. J Am Heart Assoc. 2021. PMID: 34612048 Free PMC article.
-
Association of Antihypertensives That Stimulate vs Inhibit Types 2 and 4 Angiotensin II Receptors With Cognitive Impairment.JAMA Netw Open. 2022 Jan 4;5(1):e2145319. doi: 10.1001/jamanetworkopen.2021.45319. JAMA Netw Open. 2022. PMID: 35089354 Free PMC article.
References
-
- Chobanian AV, Bakris GL, Black HR, et al. ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi:10.1001/jama.289.19.2560 - DOI - PubMed
-
- Officers A; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial . Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. doi:10.1001/jama.288.23.2981 - DOI - PubMed
-
- Leenen FHH, Nwachuku CE, Black HR, et al. ; Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group . Clinical events in high-risk hypertensive patients randomly assigned to calcium channel blocker versus angiotensin-converting enzyme inhibitor in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension. 2006;48(3):374-384. doi:10.1161/01.HYP.0000231662.77359.de - DOI - PubMed
-
- Dahlöf B, Devereux RB, Kjeldsen SE, et al. ; LIFE Study Group . Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint Reduction in Hypertension Study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995-1003. doi:10.1016/S0140-6736(02)08089-3 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

