miR-146a regulates insulin sensitivity via NPR3
- PMID: 33206203
- PMCID: PMC8004521
- DOI: 10.1007/s00018-020-03699-1
miR-146a regulates insulin sensitivity via NPR3
Abstract
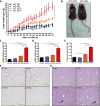
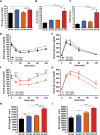
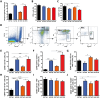
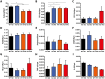
The pathogenesis of obesity-related metabolic diseases has been linked to the inflammation of white adipose tissue (WAT), but the molecular interconnections are still not fully understood. MiR-146a controls inflammatory processes by suppressing pro-inflammatory signaling pathways. The aim of this study was to characterize the role of miR-146a in obesity and insulin resistance. MiR-146a-/- mice were subjected to a high-fat diet followed by metabolic tests and WAT transcriptomics. Gain- and loss-of-function studies were performed using human Simpson-Golabi-Behmel syndrome (SGBS) adipocytes. Compared to controls, miR-146a-/- mice gained significantly more body weight on a high-fat diet with increased fat mass and adipocyte hypertrophy. This was accompanied by exacerbated liver steatosis, insulin resistance, and glucose intolerance. Likewise, adipocytes transfected with an inhibitor of miR-146a displayed a decrease in insulin-stimulated glucose uptake, while transfecting miR-146a mimics caused the opposite effect. Natriuretic peptide receptor 3 (NPR3) was identified as a direct target gene of miR-146a in adipocytes and CRISPR/Cas9-mediated knockout of NPR3 increased insulin-stimulated glucose uptake and enhanced de novo lipogenesis. In summary, miR-146a regulates systemic and adipocyte insulin sensitivity via downregulation of NPR3.
Keywords: Adipocyte; Insulin resistance; NPR3; microRNA.
Conflict of interest statement
The authors of this study have no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

