SB8: A Bevacizumab Biosimilar
- PMID: 33206282
- PMCID: PMC7733936
- DOI: 10.1007/s11523-020-00776-0
SB8: A Bevacizumab Biosimilar
Erratum in
-
Correction to: SB8: A Bevacizumab Biosimilar.Target Oncol. 2021 Jan;16(1):119. doi: 10.1007/s11523-020-00783-1. Target Oncol. 2021. PMID: 33306171 Free PMC article. No abstract available.
Abstract
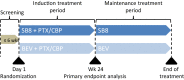
SB8 is a biosimilar of the monoclonal anti-VEGF antibody bevacizumab and is approved in the EU for use in the same types of cancer as bevacizumab. SB8 has similar physicochemical and pharmacodynamic properties to those of reference bevacizumab and pharmacokinetic equivalence was shown in healthy volunteers and patients with non-small cell lung cancer (NSCLC). SB8 demonstrated equivalent clinical efficacy to reference bevacizumab in patients with metastatic or recurrent nonsquamous NSCLC, with similar tolerability, safety and immunogenicity profiles.
Conflict of interest statement
Yahiya Syed is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Figures
Similar articles
-
A phase III, randomized, double-blind, multicenter study to compare the efficacy, safety, pharmacokinetics, and immunogenicity between SB8 (proposed bevacizumab biosimilar) and reference bevacizumab in patients with metastatic or recurrent nonsquamous non-small cell lung cancer.Lung Cancer. 2020 Aug;146:12-18. doi: 10.1016/j.lungcan.2020.05.027. Epub 2020 May 28. Lung Cancer. 2020. PMID: 32502923 Clinical Trial.
-
SB8, an approved bevacizumab biosimilar based on totality of evidence: scientific justification of extrapolation.Future Oncol. 2023 Feb;19(6):427-450. doi: 10.2217/fon-2022-1273. Epub 2023 Mar 8. Future Oncol. 2023. PMID: 36883661 Review.
-
FKB238: A Bevacizumab Biosimilar.Clin Drug Investig. 2021 Sep;41(9):825-828. doi: 10.1007/s40261-021-01065-y. Epub 2021 Aug 4. Clin Drug Investig. 2021. PMID: 34347284 Review.
-
A phase I, randomized, single-dose pharmacokinetic study comparing sb8 (bevacizumab biosimilar) with reference bevacizumab in healthy volunteers.Cancer Chemother Pharmacol. 2020 Oct;86(4):567-575. doi: 10.1007/s00280-020-04144-7. Epub 2020 Sep 19. Cancer Chemother Pharmacol. 2020. PMID: 32949267 Free PMC article. Clinical Trial.
-
MYL-1402O: A Bevacizumab Biosimilar.Target Oncol. 2022 Jan;17(1):85-88. doi: 10.1007/s11523-021-00858-7. Epub 2021 Dec 15. Target Oncol. 2022. PMID: 34910269 Free PMC article. Review.
Cited by
-
Evaluate the value of prolonging the duration of tiopronin for injection administration in preventing hepatotoxicity.Sci Rep. 2024 Feb 14;14(1):3674. doi: 10.1038/s41598-024-54314-3. Sci Rep. 2024. PMID: 38351216 Free PMC article.
-
VEGF as a Key Actor in Recurrent Respiratory Papillomatosis: A Narrative Review.Curr Issues Mol Biol. 2024 Jul 1;46(7):6757-6768. doi: 10.3390/cimb46070403. Curr Issues Mol Biol. 2024. PMID: 39057045 Free PMC article. Review.
References
-
- European Medicines Agency. Aybintio 25 mg/ml concentrate for solution for infusion: summary of product characteristics. https://www.ema.europa.eu/ (2020). Accessed 4 Sep 2020.
-
- European Medicines Agency. Aybintio: assessment report. https://www.ema.europa.eu/ (2020). Accessed 4 Sep 2020.
-
- Reck M, Luft A, Bondarenko I, et al. A phase III, randomized, double-blind, multicenter study to compare the efficacy, safety, pharmacokinetics, and immunogenicity between SB8 (proposed bevacizumab biosimilar) and reference bevacizumab in patients with metastatic or recurrent nonsquamous non-small cell lung cancer. Lung Cancer. 2020;146:12–18. doi: 10.1016/j.lungcan.2020.05.027. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources