Diastereo-, Enantio-, and anti- Selective Formation of Secondary Alcohol and Quaternary Carbon Stereocenters by Cu-Catalyzed Additions of B-Substituted Allyl Nucleophiles to Carbonyls
- PMID: 33206543
- PMCID: PMC7797407
- DOI: 10.1021/acs.orglett.0c03495
Diastereo-, Enantio-, and anti- Selective Formation of Secondary Alcohol and Quaternary Carbon Stereocenters by Cu-Catalyzed Additions of B-Substituted Allyl Nucleophiles to Carbonyls
Abstract
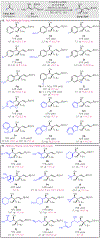
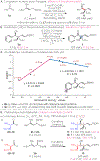
A general method for the synthesis of secondary homoallylic alcohols containing α-quaternary carbon stereogenic centers in high diastereo- and enantioselectivity (up to >20:1 dr and >99:1 er) is disclosed. Transformations employ readily accessible aldehydes, allylic diboronates, and a chiral copper catalyst and proceed by γ-addition of in situ generated enantioenriched boron-stabilized allylic copper nucleophiles. The catalytic protocol is general for a wide variety of aldehydes as well as a variety of 1,1-allylic diboronic esters. Hammett studies disclose that diastereoselectivity of the reaction is correlated to the electronic nature of the aldehyde, with dr increasing as aldehydes become more electron poor.
Conflict of interest statement
Authors declare no competing financial interests.
Figures




References
-
-
For recent reviews on the enantioselective synthesis of quaternary carbon stereogenic centers, see:
- Das JP; Marek I Enantioselective Synthesis of All-Carbon Quaternary Stereogenic Centers in Acyclic Systems. Chem. Commun 2011, 47, 4593–4623. - PubMed
- Minko Y; Marek I Stereodefined Acyclic Trisubstituted Metal Enolates towards the Asymmetric Formation of Quaternary Carbon Stereocentres. Chem Commun 2014, 50, 12597–12611. - PubMed
- Marek I; Minko Y; Pasco M; Mejuch T; Gilboa N; Chechik H; Das JP All-Carbon Quaternary Stereogenic Centers in Acyclic Systems through the Creation of Several C–C Bonds per Chemical Step. J. Am. Chem. Soc 2014, 136, 2682–2694. - PubMed
- Quasdorf KW; Overman LE Catalytic Enantioselective Synthesis of Quaternary Carbon Stereocenters. Nature 2014, 516, 181–191. - PMC - PubMed
- Liu Y; Han S-J; Liu W-B; Stoltz BM Catalytic Enantioselective Construction of Quaternary Stereocenters: Assembly of Key Building Blocks for the Synthesis of Biologically Active Molecules. Acc. Chem. Res 2015, 48, 740–751. - PMC - PubMed
- Zeng X-P; Cao Z-Y; Wang Y-H; Zhou F; Zhou J Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev 2016, 116, 7330–7396. - PubMed
- Feng J; Holmes M; Krische MJ Acyclic Quaternary Carbon Stereocenters via Enantioselective Transition Metal Catalysis. Chem. Rev 2017, 117, 12564–12580. - PMC - PubMed
-
-
-
For a review on enantioselective allylation of carbonyl compounds, including crotylation of aldehydes, see:
- Yus M; González-Gómez JC; Foubelo F Catalytic Enantioselective Allylation of Carbonyl Compounds and Imines. Chem. Rev 2011, 111, 7774–7854. - PubMed
-
-
-
For reviews of reductive couplings with aldehydes, including crotylation, see:
- Kim SW; Zhang W; Krishe MJ Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol-Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier. Acc. Chem. Res - PMC - PubMed
- Holmes M; Schwartz LA; Krische MJ Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev 2018, 118, 6026–6052. - PMC - PubMed
-
-
-
For representative examples of catalytic enantioselective crotylation of aldehydes, see:
- Traverse JF; Zhao Y; Hoveyda AH; Snapper ML Proline-Based N-Oxides as Readily Available and Modular Chiral Catalysts. Enantioselective Reactions of Allyltrichlorosilane with Aldehydes. Org. Lett 2005, 7, 3151–3154. - PubMed
- Rauniyar V; Zhai H; Hall DG Catalytic Enantioselective Allyl- and Crotylboration of Aldehydes Using Chiral Diol•SnCl4 Complexes. Optimization, Substrate Scope, and Mechanistic Investigations. J. Am. Chem. Soc 2008, 130, 8481–8490. - PubMed
- Zbieg JR; Yamaguchi E; McInturff EL; Krische MJ Enantioselective C-H Crotylation of Primary Alcohols via Hydrohydroxyalkylation of Butadiene. Science. 2012, 336, 324–327. - PMC - PubMed
- Meng F; Jang H; Jung B; Hoveyda AH Cu-Catalyzed Chemoselective Preparation of 2-(Pinacolato)boron-Substituted Allylcopper Complexes and their In Situ Site-, Diastereo-, and Enantioselective Additions to Aldehydes and Ketones. Angew. Chem. Int. Ed 2013, 52, 5046–5051. - PMC - PubMed
- Miura T; Nishida Y; Morimoto M; Murakami M Enantioselective Synthesis of Anti Homoallylic Alcohols from Terminal Alkynes and Aldehydes Based on Concomitant Use of a Cationic Iridium Complex and a Chiral Phosphoric Acid. J. Am. Chem. Soc 2013, 135, 11497–11500. - PubMed
- Miura T; Nishida Y; Murakami M Construction of Homoallylic Alcohols from Terminal Alkynes and Aldehydes with Installment of syn-Stereochemistry. J. Am. Chem. Soc 2014, 136, 6223–6226. - PubMed
- Li C; Shin K; Liu RY; Buchwald SL Engaging Aldehydes in CuH-Catalyzed Reductive Coupling Reactions: Stereoselective Allylation with Unactivated 1,3-Diene Pronucleophiles. Angew. Chem. Int. Ed 2019, 58, 17074–17080. - PMC - PubMed
-
-
- Brown HC; Bhat KS; Randad RS Charge Reversal of Electrophilic π-Allylpalladium Intermediates: Carbonyl Allylation by Allylic Acetates with Pd(PPh3)4-Zn. J. Org. Chem 1987, 52, 3702–3704.
- Sato M; Yamamoto Y; Hara S; Suzuki A A Stereoselective Synthesis of 3,3’-Disubstituted Allylborane Derivatives Using Haloboration Reaction and their Application for the Diastereospecific Synthesis of Homoallylic Alcohols Having Quaternary Carbon. Tetrahedron Lett. 1993, 34, 7071–7074.
- Kobayashi S; Nishio K Facile and Highly Stereoselective Synthesis of Homoallylic Alcohols Using Organosilicon Intermediates. J. Org. Chem 1994, 59, 6620–6628.
- Nishigaichi Y; Fujimoto M; Takuwa A γ-Selective Pentadienylation of Aldehydes and Ketones with Pentadienyltins by the Use of ZnCl2. Synlett. 1994, 731–732.
- Nowotny S; Tucker CE; Jubert C; Knochel P Chromium(II)-Mediated Stereodivergent Additions of Allylic Phosphates and Halides to Aldehydes. J. Org. Chem 1995, 60, 2762–2772.
- Nishigaichi Y; Takuwa A Stereospecificity in the Lewis Acid Promoted Allylation Reaction of 3,3-Disubstituted Allyltins toward Aldehydes. Tetrahedron Lett. 1999, 40, 109–112.
- Hirashita T; Kambe S; Tsuji H; Omori H; Araki S Direct Preparation of Allylic Indium(III) Reagents from Allylic Alchols via a Reductive Transmetalation of π-Allylnickel(II) with Indium(I) Iodide. J. Org. Chem 2004, 69, 5054–5059. - PubMed
- Yanagisawa A; Aoki T; Arai T Dibutyltin Oxide Catalyzed Allyl-Transfer Reaction from Tertiary Homoallylic Alcohols to Aldehydes. Synlett. 2006, 2071–2074.
- Ngai M-Y; Skucas E; Krische MJ Ruthenium Catalyzed C–C Bond Formation via Transfer Hydrogenation: Branch-Selective Reductive Coupling of Allenes to Paraformaldehyde and Higher Aldehydes. Org. Lett 2008, 10, 2705–2708. - PMC - PubMed
- Ely RJ; Morken JP Regio- and Stereoselective Ni-Catalyzed 1,4-Hydroboration of 1,3-Dienes: Access to Stereodefined (Z)-Allylboron Reagents and Derived Allylic Alcohols. J. Am. Chem. Soc 2010, 132, 2534–2535. - PMC - PubMed
- Biggs RA; Lambadaris M; Ogilvie WW Highly Diastereoselective Generation of Various 3,3-Disubstituted Allyl Boronates for the Stereospecific Construction of Quaternary Centers. Tetrahedron Lett. 2014, 55, 6085–6087.
- Nakano T; Endo K; Ukaji Y Silver-Catalyzed Allylation of Ketones and Intramolecular Cyclization through Carbene Intermediates from Cyclopropenes Under Ambient Conditions. Chem. Asian J 2016, 11, 713–721. - PubMed
- Weber F; Ballmann M; Kohlmeyer C; Hilt G Nickel-Catalyzed Double Bond Transposition of Alkenyl Boronates for in Situ syn-Selective Allylboration Reactions. Org. Lett 2016, 18, 548–551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

