Evolution of neuroinflammation across the lifespan of individuals with Down syndrome
- PMID: 33206953
- PMCID: PMC7805813
- DOI: 10.1093/brain/awaa326
Evolution of neuroinflammation across the lifespan of individuals with Down syndrome
Abstract
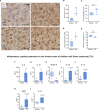
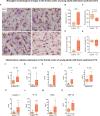
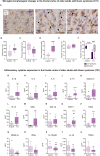
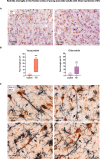
Epidemiological and experimental studies suggest that a disease-aggravating neuroinflammatory process is present at preclinical stages of Alzheimer's disease. Given that individuals with Down syndrome are at increased genetic risk of Alzheimer's disease and therefore develop the spectrum of Alzheimer's neuropathology in a uniform manner, they constitute an important population to study the evolution of neuroinflammation across the Alzheimer's continuum. Therefore, in this cross-sectional study, we characterized the brain inflammatory profile across the lifespan of individuals with Down syndrome. Microglial morphology and inflammatory cytokine expression were analysed by immunohistochemistry and electrochemiluminescent-based immunoassays in the frontal cortex from foetuses to adults with Down syndrome and control subjects (16 gestational weeks to 64 years), totalling 127 cases. Cytokine expression in mixed foetal primary cultures and hippocampus of adults with Down syndrome, as well as the effects of sex on cytokine expression were also analysed. A higher microglial soma size-to-process length ratio was observed in the frontal cortex of children and young adults with Down syndrome before the development of full-blown Alzheimer's pathology. Moreover, young adults with Down syndrome also displayed increased numbers of rod-like microglia. Increased levels of interleukin-8 and interleukin-10 were observed in children with Down syndrome (1-10 years; Down syndrome n = 5, controls n = 10) and higher levels of interleukin-1β, interleukin-1α, interleukin-6, interleukin-8, interleukin-10, interleukin-15, eotaxin-3, interferon gamma-induced protein 10, macrophage-derived chemokine, and macrophage inflammatory protein-beta, were found in young adults with Down syndrome compared to euploid cases (13-25 years, Down syndrome n = 6, controls n = 24). Increased cytokine expression was also found in the conditioned media of mixed cortical primary cultures from second trimester foetuses with Down syndrome (Down syndrome n = 7, controls n = 7). Older adults with Down syndrome (39-68 years, Down syndrome n = 22, controls n = 16) displayed reduced levels of interleukin-10, interleukin-12p40, interferon-gamma and tumour necrosis factor-alpha. Microglia displayed larger somas and shorter processes. Moreover, an increase in dystrophic microglia and rod-like microglia aligning to neurons harbouring tau pathology were also observed. Sex stratification analyses revealed that females with Down syndrome had increased interleukin-6 and interleukin-8 levels compared to males with Down syndrome. Finally, multivariate projection methods identified specific cytokine patterns among individuals with Down syndrome. Our findings indicate the presence of an early and evolving neuroinflammatory phenotype across the lifespan in Down syndrome, a knowledge that is relevant for the discovery of stage-specific targets and for the design of possible anti-inflammatory trials against Alzheimer's disease in this population.
Keywords: Alzheimer’s disease; Down syndrome; beta-amyloid; inflammation; microglia.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, Gao X, et al.NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature 2006; 441: 595–600. - PubMed

