Severe congenital neutropenia-associated JAGN1 mutations unleash a calpain-dependent cell death programme in myeloid cells
- PMID: 33206996
- PMCID: PMC7839451
- DOI: 10.1111/bjh.17137
Severe congenital neutropenia-associated JAGN1 mutations unleash a calpain-dependent cell death programme in myeloid cells
Abstract
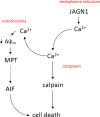
Severe congenital neutropenia (SCN) of autosomal recessive inheritance, also known as Kostmann disease, is characterised by a lack of neutrophils and a propensity for life-threatening infections. Using whole-exome sequencing, we identified homozygous JAGN1 mutations (p.Gly14Ser and p.Glu21Asp) in three patients with Kostmann-like SCN, thus confirming the recent attribution of JAGN1 mutations to SCN. Using the human promyelocytic cell line HL-60 as a model, we found that overexpression of patient-derived JAGN1 mutants, but not silencing of JAGN1, augmented cell death in response to the pro-apoptotic stimuli, etoposide, staurosporine, and thapsigargin. Furthermore, cells expressing mutant JAGN1 were remarkably susceptible to agonists that normally trigger degranulation and succumbed to a calcium-dependent cell death programme. This mode of cell death was completely prevented by pharmacological inhibition of calpain but unaffected by caspase inhibition. In conclusion, our results confirmed the association between JAGN1 mutations and SCN and showed that SCN-associated JAGN1 mutations unleash a calcium- and calpain-dependent cell death in myeloid cells.
Keywords: apoptosis; calcium; calpain; necroptosis; neutropenia.
© 2020 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Figures

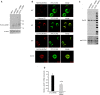
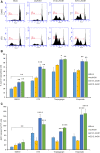
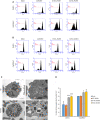
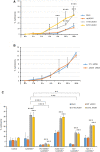
Comment in
-
JAGN1 mutations in severe congenital neutropenia.Br J Haematol. 2021 Jan;192(1):9-10. doi: 10.1111/bjh.17135. Epub 2020 Nov 18. Br J Haematol. 2021. PMID: 33207009 No abstract available.
References
-
- Donadieu J, Beaupain B, Fenneteau O, Bellanné‐Chantelot C. Congenital neutropenia in the era of genomics: classification, diagnosis, and natural history. Br J Haematol. 2017;179:557–4. - PubMed
-
- Carlsson G, Fasth A, Berglöf E, Lagerstedt‐Robinson K, Nordenskjöld M, Palmblad J, et al. Incidence of severe congenital neutropenia in Sweden and risk of evolution to myelodysplastic syndrome/leukaemia. Br J Haematol. 2012;158:363–9. - PubMed
-
- Horwitz M, Benson KF, Person RE, Aprikyan AG, Dale DC. Mutations in ELA2, encoding neutrophil elastase, define a 21‐day biological clock in cyclic haematopoiesis. Nat Genet. 1999;23:433–6. - PubMed
-
- Dale DC, Person RE, Bolyard AA, Aprikyan AG, Bos C, Bonilla MA, et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. 2000;96:2317–22. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

