BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils
- PMID: 33207187
- PMCID: PMC7672522
- DOI: 10.1016/j.celrep.2020.108387
BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils
Abstract
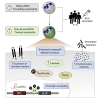
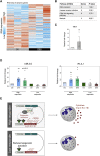
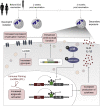
The tuberculosis vaccine bacillus Calmette-Guérin (BCG) protects against some heterologous infections, probably via induction of non-specific innate immune memory in monocytes and natural killer (NK) cells, a process known as trained immunity. Recent studies have revealed that the induction of trained immunity is associated with a bias toward granulopoiesis in bone marrow hematopoietic progenitor cells, but it is unknown whether BCG vaccination also leads to functional reprogramming of mature neutrophils. Here, we show that BCG vaccination of healthy humans induces long-lasting changes in neutrophil phenotype, characterized by increased expression of activation markers and antimicrobial function. The enhanced function of human neutrophils persists for at least 3 months after vaccination and is associated with genome-wide epigenetic modifications in trimethylation at histone 3 lysine 4. Functional reprogramming of neutrophils by the induction of trained immunity might offer novel therapeutic strategies in clinical conditions that could benefit from modulation of neutrophil effector function.
Keywords: BCG; epigenetics; innate immune memory; neutrophil; nonspecific effects of vaccines; polymorphonuclear leukocytes; trained immunity.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





References
-
- Aaby P., Shaheen S.O., Heyes C.B., Goudiaby A., Hall A.J., Shiell A.W., Jensen H., Marchant A. Early BCG vaccination and reduction in atopy in Guinea-Bissau. Clin. Exp. Allergy. 2000;30:644–650. - PubMed
-
- Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.M., Stensballe L., Diness B.R., Lausch K.R., Lund N. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011;204:245–252. - PubMed
-
- Adrover J.M., Del Fresno C., Crainiciuc G., Cuartero M.I., Casanova-Acebes M., Weiss L.A., Huerga-Encabo H., Silvestre-Roig C., Rossaint J., Cossío I. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity. 2019;50:390–402.e10. - PubMed
-
- Akashi K., Traver D., Miyamoto T., Weissman I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

