Cell Surface Mechanics Gate Embryonic Stem Cell Differentiation
- PMID: 33207217
- PMCID: PMC7875094
- DOI: 10.1016/j.stem.2020.10.017
Cell Surface Mechanics Gate Embryonic Stem Cell Differentiation
Abstract
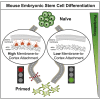
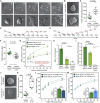
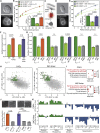
Cell differentiation typically occurs with concomitant shape transitions to enable specialized functions. To adopt a different shape, cells need to change the mechanical properties of their surface. However, whether cell surface mechanics control the process of differentiation has been relatively unexplored. Here we show that membrane mechanics gate exit from naive pluripotency of mouse embryonic stem cells. By measuring membrane tension during early differentiation, we find that naive stem cells release their plasma membrane from the underlying actin cortex when transitioning to a primed state. By mechanically tethering the plasma membrane to the cortex by enhancing Ezrin activity or expressing a synthetic signaling-inert linker, we demonstrate that preventing this detachment forces stem cells to retain their naive pluripotent identity. We thus identify a decrease in membrane-to-cortex attachment as a new cell-intrinsic mechanism that is essential for stem cells to exit pluripotency.
Keywords: atomic force spectroscopy; exit from pluripotency; mESC; membrane tension; membrane-to-cortex attachment (MCA); naive-to-primed transition.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
Comment in
-
Membrane Tension Locks In Pluripotency.Cell Stem Cell. 2021 Feb 4;28(2):175-176. doi: 10.1016/j.stem.2021.01.008. Cell Stem Cell. 2021. PMID: 33545073
References
-
- Aguilar A., Pertuy F., Eckly A., Strassel C., Collin D., Gachet C., Lanza F., Léon C. Importance of environmental stiffness for megakaryocyte differentiation and proplatelet formation. Blood. 2016;128:2022–2032. - PubMed
-
- Brons I.G.M., Smithers L.E., Trotter M.W.B., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A., Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

