Mechanical Tension Promotes Formation of Gastrulation-like Nodes and Patterns Mesoderm Specification in Human Embryonic Stem Cells
- PMID: 33207224
- PMCID: PMC7755684
- DOI: 10.1016/j.devcel.2020.10.015
Mechanical Tension Promotes Formation of Gastrulation-like Nodes and Patterns Mesoderm Specification in Human Embryonic Stem Cells
Abstract
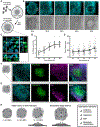
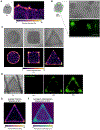
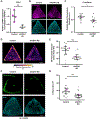
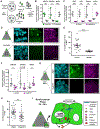
Embryogenesis is directed by morphogens that induce differentiation within a defined tissue geometry. Tissue organization is mediated by cell-cell and cell-extracellular matrix (ECM) adhesions and is modulated by cell tension and tissue-level forces. Whether cell tension regulates development by modifying morphogen signaling is less clear. Human embryonic stem cells (hESCs) exhibit an intrinsic capacity for self-organization, which motivates their use as a tractable model of early human embryogenesis. We engineered patterned substrates that recapitulate the biophysical properties of the early embryo and mediate the self-organization of "gastrulation-like" nodes in cultured hESCs. Tissue geometries that generated local nodes of high cell-adhesion tension directed the spatial patterning of the BMP4-dependent "gastrulation-like" phenotype by enhancing phosphorylation and junctional release of β-catenin to promote Wnt signaling and mesoderm specification. Furthermore, direct force application via mechanical stretching promoted BMP-dependent mesoderm specification, confirming that tissue-level forces can directly regulate cell fate specification in early human development.
Keywords: cytoskeletal tension; gastrulation; human embryonic stem cells; mesoderm; polyacrylamide hydrogels; self-organization; tissue patterning; traction force microscopy.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



Comment in
-
Cells under Tension Drive Gastrulation.Dev Cell. 2020 Dec 21;55(6):669-670. doi: 10.1016/j.devcel.2020.11.023. Dev Cell. 2020. PMID: 33352138
References
-
- Beccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy AC, Lutolf MP, Duboule D, Arias AM, 2018. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562(7726), 272–276. - PubMed
-
- Berg S, Kutra D, Kroeger T, Straehle CN, Kausler BX, Haubold C, Schiegg M, Ales J, Beier T, Rudy M, Eren K, Cervantes JI, Xu B, Beuttenmueller F, Wolny A, Zhang C, Koethe U, Hamprecht FA, Kreshuk A, 2019. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods. 1–7. - PubMed
-
- Bertocchini F, Stern CD, 2002. The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling. Dev. Cell. 3(5), 735–744. - PubMed
-
- Bienz M, 2005. β-catenin: A pivot between cell adhesion and Wnt signalling. Curr. Biol. 15(2), R64–R67. - PubMed
-
- Biguri A, 2020. Perceptually uniform colormaps. (https://www.mathworks.com/matlabcentral/fileexchange/51986-perceptually-...), MATLAB Central File Exchange; Retrieved April 24, 2020.

