Recurrent limbic seizures do not cause hippocampal neuronal loss: A prolonged laboratory study
- PMID: 33207277
- PMCID: PMC7855788
- DOI: 10.1016/j.nbd.2020.105183
Recurrent limbic seizures do not cause hippocampal neuronal loss: A prolonged laboratory study
Abstract
Purpose: It remains controversial whether neuronal damage and synaptic reorganization found in some forms of epilepsy are the result of an initial injury and potentially contributory to the epileptic condition or are the cumulative affect of repeated seizures. A number of reports of human and animal pathology suggest that at least some neuronal loss precedes the onset of seizures, but there is debate over whether there is further damage over time from intermittent seizures. In support of this latter hypothesis are MRI studies in people that show reduced hippocampal volumes and cortical thickness with longer durations of the disease. In this study we addressed the question of neuronal loss from intermittent seizures using kindled rats (no initial injury) and rats with limbic epilepsy (initial injury).
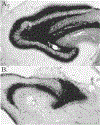
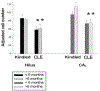
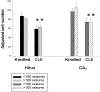
Methods: Supragranular mossy fiber sprouting, hippocampal neuronal densities, and subfield area measurements were determined in rats with chronic limbic epilepsy (CLE) that developed following an episode of limbic status epilepticus (n = 25), in kindled rats (n = 15), and in age matched controls (n = 20). To determine whether age or seizure frequency played a role in the changes, CLE and kindled rats were further classified by seizure frequency (low/high) and the duration of the seizure disorder (young/old).
Results: Overall there was no evidence for progressive neuronal loss from recurrent seizures. Compared with control and kindled rats, CLE animals showed increased mossy fiber sprouting, decreased neuronal numbers in multiple regions and regional atrophy. In CLE, but not kindled rats: 1) Higher seizure frequency was associated with greater mossy fiber sprouting and granule cell dispersion; and 2) greater age with seizures was associated with decreased hilar densities, and increased hilar areas. There was no evidence for progressive neuronal loss, even with more than 1000 seizures.
Conclusion: These findings suggest that the neuronal loss associated with limbic epilepsy precedes the onset of the seizures and is not a consequence of recurrent seizures. However, intermittent seizures do cause other structural changes in the brain, the functional consequences of which are unclear.
Keywords: Axon plasticity; Epilepsy pathology; Epileptogenesis; Mossy fibers; Pathogenesis; Temporal lobe epilepsy.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Adams B, Von Ling E, Vaccarella L, Ivy GO, Fahnestock M, Racine RJ, 1998. Time course for kindling-induced changes in the hilar area of the dentate gyrus: reactive gliosis as a potential mechanism. Brain Res 804, 331–336. - PubMed
-
- Babb TL, Brown WJ, 1987. Pathological findings in epilepsy In: Engel J Jr. (Ed.), Surgical Treatment of the Epilepsies. Raven Press, New York, pp. 511–540.
-
- Bertram EH, 1997. Functional anatomy of spontaneous seizures in a rat model of limbic epilepsy. Epilepsia 38, 95–105. - PubMed
-
- Bertram EH 3rd, Lothman EW, 1993. Morphometric effects of intermittent kindled seizures and limbic status epilepticus in the dentate gyrus of the rat. Brain Res 603, 25–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

