Engineering Next-Generation CAR-T Cells for Better Toxicity Management
- PMID: 33207607
- PMCID: PMC7696189
- DOI: 10.3390/ijms21228620
Engineering Next-Generation CAR-T Cells for Better Toxicity Management
Abstract
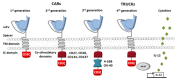
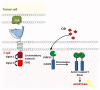
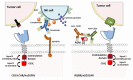
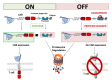
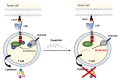
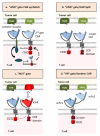
Immunoadoptive therapy with genetically modified T lymphocytes expressing chimeric antigen receptors (CARs) has revolutionized the treatment of patients with hematologic cancers. Although clinical outcomes in B-cell malignancies are impressive, researchers are seeking to enhance the activity, persistence, and also safety of CAR-T cell therapy-notably with a view to mitigating potentially serious or even life-threatening adverse events like on-target/off-tumor toxicity and (in particular) cytokine release syndrome. A variety of safety strategies have been developed by replacing or adding various components (such as OFF- and ON-switch CARs) or by combining multi-antigen-targeting OR-, AND- and NOT-gate CAR-T cells. This research has laid the foundations for a whole new generation of therapeutic CAR-T cells. Here, we review the most promising CAR-T cell safety strategies and the corresponding preclinical and clinical studies.
Keywords: CAR-T cell; cancer; chimeric antigen receptor; cytokine release syndrome; engineering; immunotherapy; toxicity.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures



References
-
- Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

