Induction of Lysosomal Membrane Permeabilization Is a Major Event of FTY720-Mediated Non-Apoptotic Cell Death in Human Glioma Cells
- PMID: 33207629
- PMCID: PMC7696845
- DOI: 10.3390/cancers12113388
Induction of Lysosomal Membrane Permeabilization Is a Major Event of FTY720-Mediated Non-Apoptotic Cell Death in Human Glioma Cells
Abstract
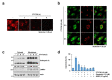
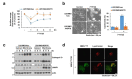
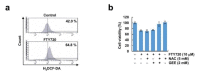
FTY720, a sphingosine-1-phosphate (S1P) receptor modulator, is a synthetic compound produced by the modification of a metabolite from I. sinclairii. Here, we found that FTY720 induced non-apoptotic cell death in human glioma cells (U251MG, U87MG, and U118MG). FTY720 (10 µM) dramatically induced cytoplasmic vacuolation in glioma cells. However, FTY720-mediated vacuolation and cell death are not associated with autophagy. Genetic or pharmacological inhibition of autophagy did not inhibit FTY720-induced cell death. Herein, we detected that FTY720-induced cytoplasmic vacuoles were stained with lysotracker red, and FTY720 induced lysosomal membrane permeabilization (LMP). Interestingly, cathepsin inhibitors (E64D and pepstatin A) and ectopic expression of heat shock protein 70 (HSP70), which is an endogenous inhibitor of LMP, markedly inhibited FTY720-induced cell death. Our results demonstrated that FTY720 induced non-apoptotic cell death via the induction of LMP in human glioma cells.
Keywords: FTY720; LMP; cathepsins; glioma; non-apoptotic cell death.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Permpongkosol S., Wang J.D., Takahara S., Matsumiya K., Nonomura N., Nishimura K., Tsujimura A., Kongkanand A., Okuyama A. Anticarcinogenic effect of FTY720 in human prostate carcinoma DU145 cells: Modulation of mitogenic signaling, FAK, cell-cycle entry and apoptosis. Int. J. Cancer. 2002;98:167–172. doi: 10.1002/ijc.10178. - DOI - PubMed
-
- Ota K., Okuma T., Lorenzo A., Yokota A., Hino H., Kazama H., Moriya S., Takano N., Hiramoto M., Miyazawa K. Fingolimod sensitizes EGFR wildtype nonsmall cell lung cancer cells to lapatinib or sorafenib and induces cell cycle arrest. Oncol. Rep. 2019;42:231–242. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

