Inactivation of H+-ATPase Participates in the Influence of Variation Potential on Photosynthesis and Respiration in Peas
- PMID: 33207655
- PMCID: PMC7697462
- DOI: 10.3390/plants9111585
Inactivation of H+-ATPase Participates in the Influence of Variation Potential on Photosynthesis and Respiration in Peas
Abstract
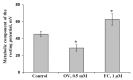
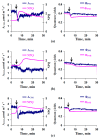
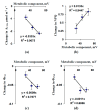
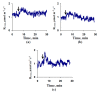
Local damage (e.g., burning, heating, or crushing) causes the generation and propagation of a variation potential (VP), which is a unique electrical signal in higher plants. A VP influences numerous physiological processes, with photosynthesis and respiration being important targets. VP generation is based on transient inactivation of H+-ATPase in plasma membrane. In this work, we investigated the participation of this inactivation in the development of VP-induced photosynthetic and respiratory responses. Two- to three-week-old pea seedlings (Pisum sativum L.) and their protoplasts were investigated. Photosynthesis and respiration in intact seedlings were measured using a GFS-3000 gas analyzer, Dual-PAM-100 Pulse-Amplitude-Modulation (PAM)-fluorometer, and a Dual-PAM gas-exchange Cuvette 3010-Dual. Electrical activity was measured using extracellular electrodes. The parameters of photosynthetic light reactions in protoplasts were measured using the Dual-PAM-100; photosynthesis- and respiration-related changes in O2 exchange rate were measured using an Oxygraph Plus System. We found that preliminary changes in the activity of H+-ATPase in the plasma membrane (its inactivation by sodium orthovanadate or activation by fusicoccin) influenced the amplitudes and magnitudes of VP-induced photosynthetic and respiratory responses in intact seedlings. Decreases in H+-ATPase activity (sodium orthovanadate treatment) induced fast decreases in photosynthetic activity and increases in respiration in protoplasts. Thus, our results support the effect of H+-ATPase inactivation on VP-induced photosynthetic and respiratory responses.
Keywords: H+-ATPase; electrical signals; pea; photosynthesis; protoplasts; respiration; systemic physiological response; variation potential.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Trebacz K., Dziubinska H., Krol E. Electrical signals in long-distance communication in plants. In: Baluška F., Mancuso S., Volkmann D., editors. Communication in Plants. Neuronal Aspects of Plant Life. Springer; Berlin/Heidelberg, Germany: New York, NY, USA: 2006. pp. 277–290.
-
- Gallé A., Lautner S., Flexas J., Fromm J. Environmental stimuli and physiological responses: The current view on electrical signaling. Environ. Exp. Bot. 2015;114:15–21. doi: 10.1016/j.envexpbot.2014.06.013. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

