Mechanochemical P-derivatization of 1,3,5-Triaza-7-Phosphaadamantane (PTA) and Silver-Based Coordination Polymers Obtained from the Resulting Phosphabetaines
- PMID: 33207789
- PMCID: PMC7697749
- DOI: 10.3390/molecules25225352
Mechanochemical P-derivatization of 1,3,5-Triaza-7-Phosphaadamantane (PTA) and Silver-Based Coordination Polymers Obtained from the Resulting Phosphabetaines
Abstract
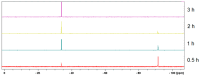
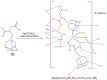
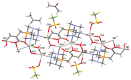
We have described earlier that in aqueous solutions, the reaction of 1,3,5-triaza-7-phosphaadamantane (PTA) with maleic acid yielded a phosphonium-alkanoate zwitterion. The same reaction with 2-methylmaleic acid (citraconic acid) proceeded much slower. It is reported here, that in the case of glutaconic and itaconic acids (constitutional isomers of citraconic acid), formation of the corresponding phosphabetaines requires significantly shorter reaction times. The new phosphabetaines were isolated and characterized by elemental analysis, multinuclear NMR spectroscopy and ESI-MS spectrometry. Furthermore, their molecular structures in the solid state were determined by single crystal X-ray diffraction (SC-XRD). Synthesis of the phosphabetaines from PTA and unsaturated dicarboxylic acids was also carried out mechanochemically with the use of a planetary ball mill, and the characteristics of the syntheses in solvent and under solvent-free conditions were compared. In aqueous solutions, the reaction of the new phosphabetaines with Ag(CF3SO3) yielded Ag(I)-based coordination polymers. According to the SC-XRD results, in these polymers the Ag(I)-ion coordinates to the N and O donor atoms of the ligands; however, Ag(I)-Ag(I) interactions were also identified. The Ag(I)-based coordination polymer (CP1.2) formed with the glutaconyl derivative of PTA (1) showed considerable antimicrobial activity against both Gram-negative and Gram-positive bacteria and yeast strains.
Keywords: 1,3,5-Triaza-7-phosphaadamantane (PTA); antimicrobial activity; ball mill; coordination polymer; mechanochemistry; phosphonium salt; silver.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures








References
-
- Anastas P.T., Warner J.C. Green Chemistry: Theory and Practice. Oxford University Press; Oxford, UK: 1998.
-
- Tanaka K. Solvent-Free Organic Synthesis. 2nd ed. Wiley-VCH Verlag GmbH & Co. KgaA; Weinheim, Germany: 2009.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

