A "Lymphocyte MicroRNA Signature" as Predictive Biomarker of Immunotherapy Response and Plasma PD-1/PD-L1 Expression Levels in Patients with Metastatic Renal Cell Carcinoma: Pointing towards Epigenetic Reprogramming
- PMID: 33207823
- PMCID: PMC7697734
- DOI: 10.3390/cancers12113396
A "Lymphocyte MicroRNA Signature" as Predictive Biomarker of Immunotherapy Response and Plasma PD-1/PD-L1 Expression Levels in Patients with Metastatic Renal Cell Carcinoma: Pointing towards Epigenetic Reprogramming
Abstract
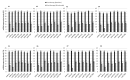
Introduction of checkpoint inhibitors resulted in durable responses and improvements in overall survival in advanced RCC patients, but the treatment efficacy is widely variable, and a considerable number of patients are resistant to PD-1/PD-L1 inhibition. This variability of clinical response makes necessary the discovery of predictive biomarkers for patient selection. Previous findings showed that the epigenetic modifications, including an extensive microRNA-mediated regulation of tumor suppressor genes, are key features of RCC. Based on this biological background, we hypothesized that a miRNA expression profile directly identified in the peripheral lymphocytes of the patients before and after the nivolumab administration could represent a step toward a real-time monitoring of the dynamic changes during cancer evolution and treatment. Interestingly, we found a specific subset of miRNAs, called "lymphocyte miRNA signature", specifically induced in long-responder patients (CR, PR, or SD to nivolumab >18 months). Focusing on the clinical translational potential of miRNAs in controlling the expression of immune checkpoints, we identified the association between the plasma levels of soluble PD-1/PD-L1 and expression of some lymphocyte miRNAs. These findings could help the development of novel dynamic predictive biomarkers urgently needed to predict the potential response to immunotherapy and to guide clinical decision-making in RCC patients.
Keywords: PD-1; PD-L1; miRNA; microRNA; predictive biomarkers; renal cell carcinoma; soluble immune checkpoints.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1 predict response to nivolumab treatment in patients with metastatic renal cell carcinoma: a step toward a biomarker for therapeutic decisions.Oncoimmunology. 2020 Oct 27;9(1):1832348. doi: 10.1080/2162402X.2020.1832348. Oncoimmunology. 2020. PMID: 33178494 Free PMC article.
-
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis.JAMA Netw Open. 2019 Jul 3;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879. JAMA Netw Open. 2019. PMID: 31290993 Free PMC article.
-
A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study.Lancet Oncol. 2018 Sep;19(9):1180-1191. doi: 10.1016/S1470-2045(18)30413-3. Epub 2018 Aug 14. Lancet Oncol. 2018. PMID: 30120041
-
The Identification of Immunological Biomarkers in Kidney Cancers.Front Oncol. 2018 Nov 2;8:456. doi: 10.3389/fonc.2018.00456. eCollection 2018. Front Oncol. 2018. PMID: 30450335 Free PMC article. Review.
-
Biomarkers for PD-1/PD-L1 Blockade Therapy in Non-Small-cell Lung Cancer: Is PD-L1 Expression a Good Marker for Patient Selection?Clin Lung Cancer. 2016 Sep;17(5):350-361. doi: 10.1016/j.cllc.2016.03.011. Epub 2016 Apr 6. Clin Lung Cancer. 2016. PMID: 27137346 Review.
Cited by
-
A Review of Recent Research on the Role of MicroRNAs in Renal Cancer.Med Sci Monit. 2021 May 8;27:e930639. doi: 10.12659/MSM.930639. Med Sci Monit. 2021. PMID: 33963171 Free PMC article. Review.
-
Can circulating PD-1, PD-L1, BTN3A1, pan-BTN3As, BTN2A1 and BTLA levels enhance prognostic power of CA125 in patients with advanced high-grade serous ovarian cancer?Front Oncol. 2022 Sep 21;12:946319. doi: 10.3389/fonc.2022.946319. eCollection 2022. Front Oncol. 2022. PMID: 36212445 Free PMC article.
-
Promises and challenges in pharmacoepigenetics.Camb Prism Precis Med. 2023 Feb 9;1:e18. doi: 10.1017/pcm.2023.6. eCollection 2023. Camb Prism Precis Med. 2023. PMID: 37560024 Free PMC article. Review.
-
Biomarkers and computational models for predicting efficacy to tumor ICI immunotherapy.Front Immunol. 2024 Mar 8;15:1368749. doi: 10.3389/fimmu.2024.1368749. eCollection 2024. Front Immunol. 2024. PMID: 38524135 Free PMC article. Review.
-
Prognostic Role of Plasma PD-1, PD-L1, pan-BTN3As and BTN3A1 in Patients Affected by Metastatic Gastrointestinal Stromal Tumors: Can Immune Checkpoints Act as a Sentinel for Short-Term Survival?Cancers (Basel). 2021 Apr 27;13(9):2118. doi: 10.3390/cancers13092118. Cancers (Basel). 2021. PMID: 33925671 Free PMC article.
References
-
- Motzer R.J., Tannir N.M., McDermott D.F., Frontera O.A., Melichar B., Choueiri T.K., Plimack E.R., Barthélémy P., Porta C., George S., et al. Nivolumab Plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. - DOI - PMC - PubMed
-
- Choueiri T., Motzer R., Rini B., Haanen J., Campbell M., Venugopal B., Kollmannsberger C., Gravis-Mescam G., Uemura M., Lee J., et al. Updated Efficacy Results from the JAVELIN Renal 101 Trial: First- Line Avelumab Plus Axitinib Versus Sunitinib in Patients With Advanced Renal Cell Carcinoma. Ann. Oncol. 2020;31:1030–1039. doi: 10.1016/j.annonc.2020.04.010. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

