Defective immunometabolism pathways in cystic fibrosis macrophages
- PMID: 33208300
- PMCID: PMC8121894
- DOI: 10.1016/j.jcf.2020.10.006
Defective immunometabolism pathways in cystic fibrosis macrophages
Abstract
Background: Mitochondria play a key role in immune defense pathways, particularly for macrophages. We and others have previously demonstrated that cystic fibrosis (CF) macrophages exhibit weak autophagy activity and exacerbated inflammatory responses. Previous studies have revealed that mitochondria are defective in CF epithelial cells, but to date, the connection between defective mitochondrial function and CF macrophage immune dysregulation has not been fully elucidated. Here, we present a characterization of mitochondrial dysfunction in CF macrophages.
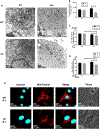
Methods: Mitochondrial function in wild-type (WT) and CF F508del/F508del murine macrophages was measured using the Seahorse Extracellular Flux analyzer. Mitochondrial morphology was investigated using transmission electron and confocal microscopy. Mitochondrial membrane potential (MMP) as well as mitochondrial reactive oxygen species (mROS) were measured using TMRM and MitoSOX Red fluorescent dyes, respectively. All assays were performed at baseline and following infection by Burkholderia cenocepacia, a multi-drug resistant bacterium that causes detrimental infections in CF patients.
Results: We have identified impaired oxygen consumption in CF macrophages without and with B. cenocepacia infection. We also observed increased mitochondrial fragmentation in CF macrophages following infection. Lastly, we observed increased MMP and impaired mROS production in CF macrophages following infection with B. cenocepacia.
Conclusions: The mitochondrial defects identified are key components of the macrophage response to infection. Their presence suggests that mitochondrial dysfunction contributes to impaired bacterial killing in CF macrophages. Our current study will enhance our understanding of the pathobiology of CF and lead to the identification of novel mitochondrial therapeutic targets for CF.
Keywords: Bacterial infection; Immunometabolism; Macrophage; Mitochondria; Reactive oxygen species.
Copyright © 2020. Published by Elsevier B.V.
Figures


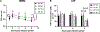

References
-
- Abdulrahman BA, Khweek AA, Akhter A, Caution K, Tazi M, Hassan H, et al.Depletion of the ubiquitin-binding adaptor molecule SQSTM1/P62 from macrophages harboring cftr Δf508 Mutation Improves the Delivery of Burkholderia cenocepacia to the Autophagic Machinery. J Biol Chem 2013;288:2049–58. doi:10.1074/jbc.M112.411728. - DOI - PMC - PubMed
-
- Elborn JS, Balfour-Lynn IM, Bilton D. Respiratory disease: Infectious complications. In: Bush A, Hodson ME, Bilton D, editors. Hodson Geddes’ Cyst. Fibros. 4th ed., CRC Press; 2015, p. 205–20.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

