Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis
- PMID: 33208407
- PMCID: PMC8458090
- DOI: 10.1136/gutjnl-2020-322509
Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis
Abstract
Objective: Lipotoxic hepatocyte injury is a primary event in non-alcoholic steatohepatitis (NASH), but the mechanisms of lipotoxicity are not fully defined. Sphingolipids and free cholesterol (FC) mediate hepatocyte injury, but their link in NASH has not been explored. We examined the role of free cholesterol and sphingomyelin synthases (SMSs) that generate sphingomyelin (SM) and diacylglycerol (DAG) in hepatocyte pyroptosis, a specific form of programmed cell death associated with inflammasome activation, and NASH.
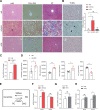
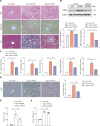
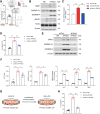
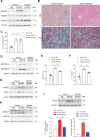
Design: Wild-type C57BL/6J mice were fed a high fat and high cholesterol diet (HFHCD) to induce NASH. Hepatic SMS1 and SMS2 expressions were examined in various mouse models including HFHCD-fed mice and patients with NASH. Pyroptosis was estimated by the generation of the gasdermin-D N-terminal fragment. NASH susceptibility and pyroptosis were examined following knockdown of SMS1, protein kinase Cδ (PKCδ), or the NLR family CARD domain-containing protein 4 (NLRC4).
Results: HFHCD increased the hepatic levels of SM and DAG while decreasing the level of phosphatidylcholine. Hepatic expression of Sms1 but not Sms2 was higher in mouse models and patients with NASH. FC in hepatocytes induced Sms1 expression, and Sms1 knockdown prevented HFHCD-induced NASH. DAG produced by SMS1 activated PKCδ and NLRC4 inflammasome to induce hepatocyte pyroptosis. Depletion of Nlrc4 prevented hepatocyte pyroptosis and the development of NASH. Conditioned media from pyroptotic hepatocytes activated the NOD-like receptor family pyrin domain containing 3 inflammasome (NLRP3) in Kupffer cells, but Nlrp3 knockout mice were not protected against HFHCD-induced hepatocyte pyroptosis.
Conclusion: SMS1 mediates hepatocyte pyroptosis through a novel DAG-PKCδ-NLRC4 axis and holds promise as a therapeutic target for NASH.
Keywords: hepatocyte; immunology in hepatology; lipid mediators; nonalcoholic steatohepatitis.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures