Sexually transmitted infections among women randomised to depot medroxyprogesterone acetate, a copper intrauterine device or a levonorgestrel implant
- PMID: 33208512
- PMCID: PMC8165154
- DOI: 10.1136/sextrans-2020-054590
Sexually transmitted infections among women randomised to depot medroxyprogesterone acetate, a copper intrauterine device or a levonorgestrel implant
Abstract
Objectives: Reproductive aged women are at risk of pregnancy and sexually transmitted infections (STI). Understanding drivers of STI acquisition, including any association with widely used contraceptives, could help us to reduce STI prevalence and comorbidities. We compared the risk of STI among women randomised to three contraceptive methods.
Methods: We conducted a secondary analysis to assess the risk of chlamydia and gonorrhoea in a clinical trial evaluating HIV risk among 7829 women aged 16-35 randomised to intramuscular depot medroxyprogesterone acetate (DMPA-IM), a copper intrauterine device (IUD) or a levonorgestrel (LNG) implant. We estimated chlamydia and gonorrhoea prevalences by contraceptive group and prevalence ratios (PR) using log-binomial regression.
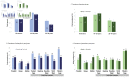
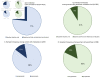
Results: At baseline, chlamydia and gonorrhoea prevalences were 18% and 5%, respectively. Final visit chlamydia prevalence did not differ significantly between DMPA-IM and copper IUD groups or between copper IUD and LNG implant groups. The DMPA-IM group had significantly lower risk of chlamydia compared with the LNG implant group (PR 0.83, 95% CI 0.72 to 0.95). Final visit gonorrhoea prevalence differed significantly only between the DMPA-IM and the copper IUD groups (PR 0.67, 95% CI 0.52 to 0.87).
Conclusions: The findings suggest that chlamydia and gonorrhoea risk may vary with contraceptive method use. Further investigation is warranted to better understand the mechanisms of chlamydia and gonorrhoea susceptibility in the context of contraceptive use.
Keywords: chlamydia trachomatis; clinical trials; contraception; neisseria gonorrhoeae.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: JMB is an advisor for Gilead Sciences, Merck and Janssen.
Figures
Similar articles
-
HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial.Lancet. 2019 Jul 27;394(10195):303-313. doi: 10.1016/S0140-6736(19)31288-7. Epub 2019 Jun 13. Lancet. 2019. PMID: 31204114 Free PMC article. Clinical Trial.
-
Sexual behaviour among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: Data from the ECHO randomized trial.PLoS One. 2024 May 9;19(5):e0299802. doi: 10.1371/journal.pone.0299802. eCollection 2024. PLoS One. 2024. PMID: 38722832 Free PMC article. Clinical Trial.
-
Contraceptive method preference and reasons for contraceptive discontinuation among women randomized to intramuscular depot medroxyprogesterone acetate, a copper intrauterine device or a levonorgestrel implant: Findings from Durban, South Africa.Contraception. 2022 Apr;108:37-43. doi: 10.1016/j.contraception.2021.11.002. Epub 2021 Nov 27. Contraception. 2022. PMID: 34848180 Clinical Trial.
-
Long-acting contraceptive options.Int J Fertil Menopausal Stud. 1996 Mar-Apr;41(2):69-76. Int J Fertil Menopausal Stud. 1996. PMID: 8829701 Review.
-
Highly effective contraception and acquisition of HIV and other sexually transmitted infections.Best Pract Res Clin Obstet Gynaecol. 2009 Apr;23(2):263-84. doi: 10.1016/j.bpobgyn.2008.11.004. Epub 2009 Feb 10. Best Pract Res Clin Obstet Gynaecol. 2009. PMID: 19211309 Review.
Cited by
-
Contraceptive effects on the cervicovaginal microbiome: Recent evidence including randomized trials.Am J Reprod Immunol. 2023 Nov;90(5):e13785. doi: 10.1111/aji.13785. Am J Reprod Immunol. 2023. PMID: 37881121 Free PMC article. Review.
-
Hormonal contraception for women at risk of HIV infection.Cochrane Database Syst Rev. 2025 Jun 6;6(6):CD015701. doi: 10.1002/14651858.CD015701.pub2. Cochrane Database Syst Rev. 2025. PMID: 40476466 Review.
-
Systems analysis reveals differential expression of endocervical genes in African women randomized to DMPA-IM, LNG implant or cu-IUD.Clin Immunol. 2023 Oct;255:109750. doi: 10.1016/j.clim.2023.109750. Epub 2023 Sep 3. Clin Immunol. 2023. PMID: 37660744 Free PMC article. Clinical Trial.
-
Implementation preferences for the management of sexually transmitted infections in the South African health system: a discrete choice experiment.Sex Transm Infect. 2024 Jan 17;100(1):10-16. doi: 10.1136/sextrans-2023-055816. Sex Transm Infect. 2024. PMID: 37918916 Free PMC article.
-
Giving voice to the end-user: input on multipurpose prevention technologies from the perspectives of young women in Kenya and South Africa.Sex Reprod Health Matters. 2021 Dec;29(1):1927477. doi: 10.1080/26410397.2021.1927477. Sex Reprod Health Matters. 2021. PMID: 34224341 Free PMC article. Clinical Trial.
References
-
- South Africa National Department of Health . South Africa demographic and health survey, 2016. Available: https://dhsprogram.com/pubs/pdf/FR337/FR337.pdf [Accessed 01 Mar 2020].
-
- Family planning.org . Family planning, 2020. Available: http://www.familyplanning2020.org/microsite/about-us [Accessed 01 Mar 2020].
-
- Torrone EA, Morrison CS, Chen P-L, et al. . Correction: prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med 2018;15:e1002608. 10.1371/journal.pmed.1002608 - DOI - PMC - PubMed
-
- World Health Organization . Global health sector strategy on sexually transmitted infections, 2016-2021. Available: https://www.who.int/reproductivehealth/publications/rtis/ghss-stis/en/ [Accessed 01 Mar 2020].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
