Hyaluronan control of the primary vascular barrier during early mouse pregnancy is mediated by uterine NK cells
- PMID: 33208556
- PMCID: PMC7710306
- DOI: 10.1172/jci.insight.135775
Hyaluronan control of the primary vascular barrier during early mouse pregnancy is mediated by uterine NK cells
Abstract
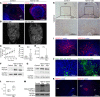
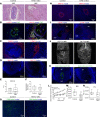
Successful implantation is associated with a unique spatial pattern of vascular remodeling, characterized by profound peripheral neovascularization surrounding a periembryo avascular niche. We hypothesized that hyaluronan controls the formation of this distinctive vascular pattern encompassing the embryo. This hypothesis was evaluated by genetic modification of hyaluronan metabolism, specifically targeted to embryonic trophoblast cells. The outcome of altered hyaluronan deposition on uterine vascular remodeling and postimplantation development were analyzed by MRI, detailed histological examinations, and RNA sequencing of uterine NK cells. Our experiments revealed that disruption of hyaluronan synthesis, as well as its increased cleavage at the embryonic niche, impaired implantation by induction of decidual vascular permeability, defective vascular sinus folds formation, breach of the maternal-embryo barrier, elevated MMP-9 expression, and interrupted uterine NK cell recruitment and function. Conversely, enhanced deposition of hyaluronan resulted in the expansion of the maternal-embryo barrier and increased diffusion distance, leading to compromised implantation. The deposition of hyaluronan at the embryonic niche is regulated by progesterone-progesterone receptor signaling. These results demonstrate a pivotal role for hyaluronan in successful pregnancy by fine-tuning the periembryo avascular niche and maternal vascular morphogenesis.
Keywords: Angiogenesis; Embryonic development; Mouse models; Reproductive Biology.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

