Assessment of Central Nervous System Lymphoma Based on CXCR4 Expression In Vivo Using 68Ga-Pentixafor PET/MRI
- PMID: 33208624
- PMCID: PMC8385649
- DOI: 10.1097/RLU.0000000000003404
Assessment of Central Nervous System Lymphoma Based on CXCR4 Expression In Vivo Using 68Ga-Pentixafor PET/MRI
Abstract
Purpose of the report: F-FDG PET is limited for assessment of central nervous system lymphoma (CNSL) due to physiologic tracer accumulation in the brain. We prospectively evaluated the novel PET tracer Ga-pentixafor, which targets the C-X-C chemokine receptor 4 (CXCR4), for lesion visualization and response assessment of CNSL.
Materials and methods: Seven CNSL patients underwent Ga-pentixafor PET/MRI with contrast enhancement (CE-MRI) and diffusion-weighted sequences. The accuracy of Ga-pentixafor PET for CNSL lesion detection relative to the CE-MRI reference standard was determined. Standardized uptake values (SUVmean and SUVmax), PET-based (PTV) and MRI-based (VOLMRI) tumor volumes, and apparent diffusion coefficients (ADCs) were assessed, and correlation coefficients were calculated. Three SUVmax thresholds (41%, 50%, and 70%) were evaluated for PTV definitions (PTV41%, PTV50%, and PTV70%) and tested against VOLMRI using paired sample t tests.
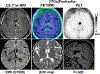
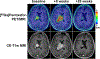
Results: Twelve Ga-pentixafor PET/MRI examinations (including 5 follow-up scans) of 7 patients were evaluated. Ga-pentixafor PET demonstrated 18 lesions, all of which were confirmed by CE-MRI; there were no false-positive lesions on PET (accuracy, 100%). PTV41% showed the highest concordance with lesion morphology, with no significant difference compared with VOLMRI (mean difference, -0.24 cm; P = 0.45). The correlation between ADCmean and SUVmean41% (r = 0.68) was moderate. Changes in PTV41% on follow-up PET/MRI showed the same trend as VOLMRI changes, including progression of 1 lesion each in patient 1 (+456.0% PTV41% and +350.8% VOLMRI) and patient 3 (+110.4% PTV41% and +85.1% VOLMRI).
Conclusions: Ga-pentixafor PET may be feasible for assessment and follow-up of CNSL. Future studies need to focus on testing its clinical value to distinguish between glioma and CNSL, and between radiation-induced inflammation and viable residual tumor.
Conflict of interest statement
Conflicts of interest:
All other authors report no conflicts of interest concerning this specific publication.
Figures
References
-
- Hoang-Xuan K, Bessell E, Bromberg J, et al.Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol [Internet]. 2015. [cited 2019];16:e322–e332. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26149884 - PubMed
-
- Cheson BD, Fisher RI, Barrington SF, et al.Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J Clin Oncol [Internet]. 2014. [cited 2019];32:3059–3067. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25113753 - PMC - PubMed
-
- Kawase Y, Yamamoto Y, Kameyama R, et al.Comparison of 11C-methionine PET and 18F-FDG PET in patients with primary central nervous system lymphoma. Mol Imaging Biol [Internet]. 2011. [cited 2020];13:1284–1289. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21042866 - PubMed
-
- Hovhannisyan N, Fillesoye F, Guillouet S, et al.[ 18 F]Fludarabine-PET as a promising tool for differentiating CNS lymphoma and glioblastoma: Comparative analysis with [ 18 F]FDG in human xenograft models. Theranostics [Internet]. 2018. [cited 2019];8:4563–4573. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30214639 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

