High-dimensional super-resolution imaging reveals heterogeneity and dynamics of subcellular lipid membranes
- PMID: 33208737
- PMCID: PMC7674432
- DOI: 10.1038/s41467-020-19747-0
High-dimensional super-resolution imaging reveals heterogeneity and dynamics of subcellular lipid membranes
Abstract
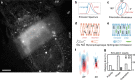
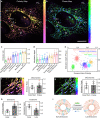
Lipid membranes are found in most intracellular organelles, and their heterogeneities play an essential role in regulating the organelles' biochemical functionalities. Here we report a Spectrum and Polarization Optical Tomography (SPOT) technique to study the subcellular lipidomics in live cells. Simply using one dye that universally stains the lipid membranes, SPOT can simultaneously resolve the membrane morphology, polarity, and phase from the three optical-dimensions of intensity, spectrum, and polarization, respectively. These high-throughput optical properties reveal lipid heterogeneities of ten subcellular compartments, at different developmental stages, and even within the same organelle. Furthermore, we obtain real-time monitoring of the multi-organelle interactive activities of cell division and successfully reveal their sophisticated lipid dynamics during the plasma membrane separation, tunneling nanotubules formation, and mitochondrial cristae dissociation. This work suggests research frontiers in correlating single-cell super-resolution lipidomics with multiplexed imaging of organelle interactome.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Harayama T, Riezman H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018;19:281–296. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

