JavaCyte, a novel open-source tool for automated quantification of key hallmarks of cardiac structural remodeling
- PMID: 33208780
- PMCID: PMC7675975
- DOI: 10.1038/s41598-020-76932-3
JavaCyte, a novel open-source tool for automated quantification of key hallmarks of cardiac structural remodeling
Abstract
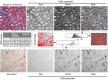
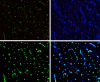
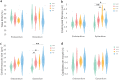
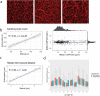
Many cardiac pathologies involve changes in tissue structure. Conventional analysis of structural features is extremely time-consuming and subject to observer bias. The possibility to determine spatial interrelations between these features is often not fully exploited. We developed a staining protocol and an ImageJ-based tool (JavaCyte) for automated histological analysis of cardiac structure, including quantification of cardiomyocyte size, overall and endomysial fibrosis, spatial patterns of endomysial fibrosis, fibroblast density, capillary density and capillary size. This automated analysis was compared to manual quantification in several well-characterized goat models of atrial fibrillation (AF). In addition, we tested inter-observer variability in atrial biopsies from the CATCH-ME consortium atrial tissue bank, with patients stratified by their cardiovascular risk profile for structural remodeling. We were able to reproduce previous manually derived histological findings in goat models for AF and AV block (AVB) using JavaCyte. Furthermore, strong correlation was found between manual and automated observations for myocyte count (r = 0.94, p < 0.001), myocyte diameter (r = 0.97, p < 0.001), endomysial fibrosis (r = 0.98, p < 0.001) and capillary count (r = 0.95, p < 0.001) in human biopsies. No significant variation between observers was observed (ICC = 0.89, p < 0.001). We developed and validated an open-source tool for high-throughput, automated histological analysis of cardiac tissue properties. JavaCyte was as accurate as manual measurements, with less inter-observer variability and faster throughput.
Conflict of interest statement
The authors declare no competing interests.
Figures