Deciphering the origin of million-fold reactivity observed for the open core diiron [HO-FeIII-O-FeIV[double bond, length as m-dash]O]2+ species towards C-H bond activation: role of spin-states, spin-coupling, and spin-cooperation
- PMID: 33209248
- PMCID: PMC7654192
- DOI: 10.1039/d0sc02624g
Deciphering the origin of million-fold reactivity observed for the open core diiron [HO-FeIII-O-FeIV[double bond, length as m-dash]O]2+ species towards C-H bond activation: role of spin-states, spin-coupling, and spin-cooperation
Abstract
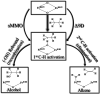
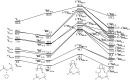
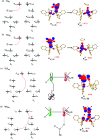
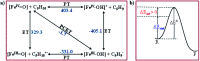
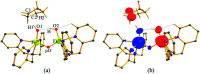
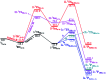
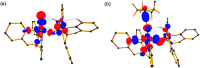
High-valent metal-oxo species have been characterised as key intermediates in both heme and non-heme enzymes that are found to perform efficient aliphatic hydroxylation, epoxidation, halogenation, and dehydrogenation reactions. Several biomimetic model complexes have been synthesised over the years to mimic both the structure and function of metalloenzymes. The diamond-core [Fe2(μ-O)2] is one of the celebrated models in this context as this has been proposed as the catalytically active species in soluble methane monooxygenase enzymes (sMMO), which perform the challenging chemical conversion of methane to methanol at ease. In this context, a report of open core [HO(L)FeIII-O-FeIV(O)(L)]2+ (1) gains attention as this activates C-H bonds a million-fold faster compared to the diamond-core structure and has the dual catalytic ability to perform hydroxylation as well as desaturation with organic substrates. In this study, we have employed density functional methods to probe the origin of the very high reactivity observed for this complex and also to shed light on how this complex performs efficient hydroxylation and desaturation of alkanes. By modelling fifteen possible spin-states for 1 that could potentially participate in the reaction mechanism, our calculations reveal a doublet ground state for 1 arising from antiferromagnetic coupling between the quartet FeIV centre and the sextet FeIII centre, which regulates the reactivity of this species. The unusual stabilisation of the high-spin ground state for FeIV[double bond, length as m-dash]O is due to the strong overlap of with the orbital, reducing the antibonding interactions via spin-cooperation. The electronic structure features computed for 1 are consistent with experiments offering confidence in the methodology chosen. Further, we have probed various mechanistic pathways for the C-H bond activation as well as -OH rebound/desaturation of alkanes. An extremely small barrier height computed for the first hydrogen atom abstraction by the terminal FeIV[double bond, length as m-dash]O unit was found to be responsible for the million-fold activation observed in the experiments. The barrier height computed for -OH rebound by the FeIII-OH unit is also smaller suggesting a facile hydroxylation of organic substrates by 1. A strong spin-cooperation between the two iron centres also reduces the barrier for second hydrogen atom abstraction, thus making the desaturation pathway competitive. Both the spin-state as well as spin-coupling between the two metal centres play a crucial role in dictating the reactivity for species 1. By exploring various mechanistic pathways, our study unveils the fact that the bridged μ-oxo group is a poor electrophile for both C-H activation as well for -OH rebound. As more and more evidence is gathered in recent years for the open core geometry of sMMO enzymes, the idea of enhancing the reactivity via an open-core motif has far-reaching consequences.
This journal is © The Royal Society of Chemistry 2020.
Figures






Similar articles
-
Interplay of Electronic Cooperativity and Exchange Coupling in Regulating the Reactivity of Diiron(IV)-oxo Complexes towards C-H and O-H Bond Activation.Chemistry. 2017 Jul 26;23(42):10110-10125. doi: 10.1002/chem.201701059. Epub 2017 Jul 5. Chemistry. 2017. PMID: 28498623
-
Comparative oxidative ability of mononuclear and dinuclear high-valent iron-oxo species towards the activation of methane: does the axial/bridge atom modulate the reactivity?Dalton Trans. 2023 Jan 3;52(2):308-325. doi: 10.1039/d2dt02559k. Dalton Trans. 2023. PMID: 36504243
-
Substrate-triggered activation of a synthetic [Fe2(μ-O)2] diamond core for C-H bond cleavage.J Am Chem Soc. 2011 Oct 19;133(41):16657-67. doi: 10.1021/ja207131g. Epub 2011 Sep 21. J Am Chem Soc. 2011. PMID: 21899336 Free PMC article.
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
-
Hydroxylation of C-H bonds at carboxylate-bridged diiron centres.Philos Trans A Math Phys Eng Sci. 2005 Apr 15;363(1829):861-77; discussion 1035-40. doi: 10.1098/rsta.2004.1532. Philos Trans A Math Phys Eng Sci. 2005. PMID: 15901540 Review.
Cited by
-
The interplay of covalency, cooperativity, and coupling strength in governing C-H bond activation in Ni2E2 (E = O, S, Se, Te) complexes.Chem Sci. 2024 Jun 3;15(27):10529-10540. doi: 10.1039/d4sc02882a. eCollection 2024 Jul 10. Chem Sci. 2024. PMID: 38994414 Free PMC article.
-
Second Coordination Sphere Effects on the Mechanistic Pathways for Dioxygen Activation by a Ferritin: Involvement of a Tyr Radical and the Identification of a Cation Binding Site.Chembiochem. 2022 Jul 5;23(13):e202200257. doi: 10.1002/cbic.202200257. Epub 2022 May 23. Chembiochem. 2022. PMID: 35510795 Free PMC article.
-
Ligand-Constraint-Induced Peroxide Activation for Electrophilic Reactivity.Angew Chem Int Ed Engl. 2021 Jun 25;60(27):14954-14959. doi: 10.1002/anie.202100438. Epub 2021 May 28. Angew Chem Int Ed Engl. 2021. PMID: 33843113 Free PMC article.
-
Structure-Spectroscopy Correlations for Intermediate Q of Soluble Methane Monooxygenase: Insights from QM/MM Calculations.J Am Chem Soc. 2021 May 5;143(17):6560-6577. doi: 10.1021/jacs.1c01180. Epub 2021 Apr 22. J Am Chem Soc. 2021. PMID: 33884874 Free PMC article.
-
Highly selective generation of singlet oxygen from dioxygen with atomically dispersed catalysts.Chem Sci. 2022 Apr 19;13(19):5606-5615. doi: 10.1039/d2sc01110g. eCollection 2022 May 18. Chem Sci. 2022. PMID: 35694341 Free PMC article.
References
-
- Ghafoor S., Mansha A., de Visser S. P. J. Am. Chem. Soc. 2019;141:20278–20292. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

