Single perineal incision for artificial urinary sphincter: analysis of technique, outcomes, and experience
- PMID: 33209655
- PMCID: PMC7658167
- DOI: 10.21037/tau-20-508
Single perineal incision for artificial urinary sphincter: analysis of technique, outcomes, and experience
Abstract
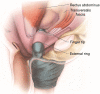
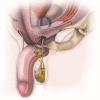
Background: To describe a large series of male patients who underwent a minimally invasive single perineal incision artificial urinary sphincter (AUS) placement in patients with stress urinary incontinence.
Methods: A retrospective cohort study was performed with data collected from men undergoing AUS placement by a single high-volume surgeon over a 12-year period (2005 to 2017). Demographic and outcomes data related to AUS placement were recorded from electronic medical records, which included subjective histories and questionnaires. Institutional ethics approval was received.
Results: A total of 145 AUS were placed over the study period. Of these, 84 were performed through a single perineal incision for both device and reservoir placement. Almost all (n=81, 96%) reported pre-operative incontinence of more than 3 pads per day. Postoperatively, 75% were satisfied with their continence, with 21 (25%) complaining of recurrent incontinence. A total of 5 (6%) patients developed a post-operative infection, 10 (12%) had device erosion and 11 (13%) had device malfunction, but only 3 (4%) had reservoir dysfunction. A total of 24 (29%) patients required revision of their device at median of 20 months (IQR, 6-32.5 months).
Conclusions: Single perineal incision is a feasible, safe, and potentially superior approach for AUS placement and warrants consideration as an accepted approach due to its more rapid surgical times, lower morbidity related to a single incision with minimal fascial defect, and favorable complication rates.
Keywords: Artificial urinary sphincter (AUS); post-prostatectomy incontinence (PPI); single incision.
2020 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-508). GB serves as an unpaid editorial board member of Translational Lung Cancer Research from Mar 2018 to Feb 2020. GB reports other from Boston Scientific, Eli Lilly, Acerus Pharma, Pfizer, Paladin, Merck, Upjohn, outside the submitted work. The authors have no other conflicts of interest to declare.
Figures

Comment in
-
Trauma, and Genital and Urethral Reconstruction.J Urol. 2021 Jul;206(1):151-152. doi: 10.1097/JU.0000000000001800. Epub 2021 Apr 20. J Urol. 2021. PMID: 33874727 No abstract available.
References
LinkOut - more resources
Full Text Sources
