LncRNA CARLo-7 facilitates proliferation, migration, invasion, and EMT of bladder cancer cells by regulating Wnt/β-catenin and JAK2/STAT3 signaling pathways
- PMID: 33209690
- PMCID: PMC7658127
- DOI: 10.21037/tau-20-1293
LncRNA CARLo-7 facilitates proliferation, migration, invasion, and EMT of bladder cancer cells by regulating Wnt/β-catenin and JAK2/STAT3 signaling pathways
Abstract
Background: Aberrant expression of long noncoding RNAs (lncRNAs) has been found to enroll in the initiation and progression of bladder cancer (BC). Earlier results show cancer-associated region long noncoding RNA-7 (CARLo-7) can be a prognostic marker for BC, but its biological function and the underlying mechanism is still to be discovered. Our study aims to explore the effects of CARLo-7 on the initiation and progression of BC and the potential mechanisms.
Methods: The expression of CARLo-7 in BC tissues and cell lines was determined by quantitative real-time polymerase chain reaction (qRT-PCR). T24 and HT1197 cells were transfected with CARLo-7 expression vector or sh-CARLo-7, then cell viability assay, BrdU assay, flow cytometry, Transwell cell migration, and invasion assay, and western blot were conducted to evaluate cell proliferation, apoptosis, invasion, migration, and epithelial-mesenchymal transition (EMT).
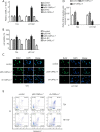
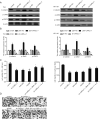
Results: CARLo-7 was dramatically upregulated in BC tissues and cell lines. Silencing CARLo-7 by sh-CARLo-7 significantly suppressed proliferation and induced apoptosis of BC cells, while enforced CARLo-7 expression promoted cell proliferation. Meanwhile, silencing CARLo-7 attenuated migration, invasion, and EMT of BC cells, while CARLo-7 overexpression had the contrary effects. The β-catenin, p-JAK2 and p-STAT3 levels were decreased by CARLo-7 knockdown, while activation of Wnt/β-catenin or JAK2/STAT3 pathways abolished the effects of CARLo-7 knockdown on cell proliferation and migration.
Conclusions: Collectively, CARLo-7 plays a critical role in regulating BC development by regulating cell proliferation, migration, invasion, and EMT through Wnt/β-catenin and JAK2/STAT3 signaling. Therefore, CARLo-7 might be a promising therapeutic target for BC.
Keywords: Cancer-associated region long noncoding RNA-7 (CARLo-7); JAK2/STAT3; Wnt/β-catenin; bladder cancer (BC); metastasis; proliferation.
2020 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1293). The authors have no conflicts of interest to declare.
Figures


Similar articles
-
The lncRNA DLX6-AS1 promoted cell proliferation, invasion, migration and epithelial-to-mesenchymal transition in bladder cancer via modulating Wnt/β-catenin signaling pathway.Cancer Cell Int. 2019 Nov 26;19:312. doi: 10.1186/s12935-019-1010-z. eCollection 2019. Cancer Cell Int. 2019. PMID: 31787849 Free PMC article.
-
Long noncoding RNA SNHG16 contributes to the development of bladder cancer via regulating miR-98/STAT3/Wnt/β-catenin pathway axis.J Cell Biochem. 2018 Nov;119(11):9408-9418. doi: 10.1002/jcb.27257. Epub 2018 Aug 21. J Cell Biochem. 2018. Retraction in: J Cell Biochem. 2021 Nov;122 Suppl 1:S23. doi: 10.1002/jcb.29982. PMID: 30132983 Retracted.
-
C19orf10 promotes malignant behaviors of human bladder carcinoma cells via regulating the PI3K/AKT and Wnt/β-catenin pathways.J Cancer. 2021 May 19;12(14):4341-4354. doi: 10.7150/jca.56993. eCollection 2021. J Cancer. 2021. PMID: 34093834 Free PMC article.
-
Long noncoding RNA H19: functions and mechanisms in regulating programmed cell death in cancer.Cell Death Discov. 2024 Feb 14;10(1):76. doi: 10.1038/s41420-024-01832-8. Cell Death Discov. 2024. PMID: 38355574 Free PMC article. Review.
-
Long Non-Coding RNA: Dual Effects on Breast Cancer Metastasis and Clinical Applications.Cancers (Basel). 2019 Nov 16;11(11):1802. doi: 10.3390/cancers11111802. Cancers (Basel). 2019. PMID: 31744046 Free PMC article. Review.
Cited by
-
The interplay between non-coding RNAs and Wnt/ß-catenin signaling pathway in urinary tract cancers: from tumorigenesis to metastasis.EXCLI J. 2022 Oct 18;21:1273-1284. doi: 10.17179/excli2022-5348. eCollection 2022. EXCLI J. 2022. PMID: 36483915 Free PMC article. Review.
-
Role of STAT3 in cancer cell epithelial‑mesenchymal transition (Review).Int J Oncol. 2024 May;64(5):48. doi: 10.3892/ijo.2024.5636. Epub 2024 Mar 15. Int J Oncol. 2024. PMID: 38488027 Free PMC article. Review.
-
Wnt/β-catenin-driven EMT regulation in human cancers.Cell Mol Life Sci. 2024 Feb 9;81(1):79. doi: 10.1007/s00018-023-05099-7. Cell Mol Life Sci. 2024. PMID: 38334836 Free PMC article. Review.
-
The role of microbiota in tumorigenesis, progression and treatment of bladder cancer.Microbiome Res Rep. 2023 Nov 20;3(1):5. doi: 10.20517/mrr.2023.47. eCollection 2024. Microbiome Res Rep. 2023. PMID: 38455086 Free PMC article. Review.
-
LINC01315 accelerates the growth and epithelial-mesenchymal transition of colorectal cancer cells via activating the Wnt/β-catenin signal.Bioengineered. 2022 Apr;13(4):8396-8406. doi: 10.1080/21655979.2022.2044275. Bioengineered. 2022. PMID: 35322763 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
