A term neonate with early myoclonic encephalopathy caused by RARS2 gene variants: a case report
- PMID: 33209735
- PMCID: PMC7658767
- DOI: 10.21037/tp-20-110
A term neonate with early myoclonic encephalopathy caused by RARS2 gene variants: a case report
Abstract
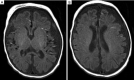
The RARS2 gene encodes mitochondrial arginine-tRNA synthetase. Patients with variants of the RARS2 gene have pontocerebellar hypoplasia type 6 (PCH6), which is characterized by early onset seizures, progressive microcephaly, and developmental delay. PCH6 is a rare mitochondrial encephalopathy. To the best of our knowledge, the onset seizure type which the ictal video-electroencephalogram (VEEG) was compatible with early myoclonic encephalopathy (EME) has not been reported. Here we reported a term female neonate with EME caused by heterozygous variants of the RARS2 gene [NM_020320: exon10: c.773G>A (p. R258H) Maternal, NM_020320: exon4: c.282_285delAGAG Paternal]. Groan was the first symptom manifested, followed by metabolic disorders, and early marked cerebral atrophy. Metabolic disorders were corrected after feeding with extensively hydrolyzed protein formula. Seizures started at the 19th day of life. Interictal VEEG showed a suppression-burst (SB) pattern and ictal VEEG revealed myoclonic seizures that were compatible with early myoclonic encephalopathy (EME). She had frequent myoclonic seizures resistant to multi-antiepileptic drugs including phenobarbital, levetiracetam and oxcarbazepine, and soon developed into convulsive status epilepticus. At 7 months of age, she had severe developmental delay, and developed infantile spasms. Our case report expands the phenotypic spectrum of the PCH6, meanwhile, RARS2 should be considered be a causative gene in patients with EME.
Keywords: Mitochondrial arginyl-tRNA synthetase 2 (RARS2); case report; early myoclonic encephalopathy (EME).
2020 Translational Pediatrics. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-110). The authors have no conflicts of interest to declare.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
