Clinical Development of Gene Therapies: The First Three Decades and Counting
- PMID: 33209964
- PMCID: PMC7658574
- DOI: 10.1016/j.omtm.2020.10.004
Clinical Development of Gene Therapies: The First Three Decades and Counting
Abstract
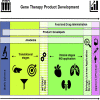
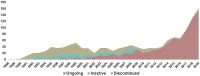
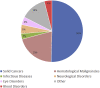
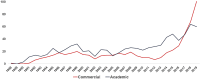
In the past three decades the field of gene therapy has made remarkable progress, surging from mere laboratory experiments to Food and Drug Administration (FDA)-approved products that bring significant reduction in disease burden to patients who previously had no therapeutic options for their serious conditions. Herein, we review the evolution of the gene therapy clinical research landscape and describe the gene therapy product development programs evaluated by the FDA in Investigational New Drug applications received in 1988-2019. We also discuss the clinical development programs of the first six oncolytic and gene therapy products approved in the United States.
Keywords: CBER; Center for Biologics Evaluation and Research; FDA; IND; Investigational New Drug application; OTAT; Office of Tissues and Advanced Therapies; gene therapy; natural history; product development; rare diseases.
Figures
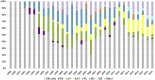
References
-
- U.S. Food and Drug Administration . 2020. Chemistry, Manufacturing, and Control Information for Human Gene Therapy Investigational New Drug Applications: Guidance for Industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
-
- National Institutes of Health. Novel and Exceptional Technology and Research Advisory Committee https://osp.od.nih.gov/biotechnology/main-nextrac/.
-
- U.S. Food and Drug Administration . 2014. Guidance Document. Expedited Programs for Serious Conditions – Drugs and Biologics.https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
-
- U.S. Food and Drug Administration . 2019. Expedited Programs for Regenerative Medicine Therapies for Serious Conditions. Guidance for Industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

